Correct Answer

verified
Correct Answer
verified
Essay
Draw the most stable conformation of trans-1,4-dipropylcyclohexane.
Correct Answer

verified
Correct Answer
verified
Essay
Place the following alkanes in order of increasing boiling point: CH3(CH2)6CH3, CH3(CH2)5CH3, (CH3)3CCH2CH2CH3
Correct Answer

verified
(CH3)3CCH2CH2C...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Arrange the following Newman projections of butane in order of energy. 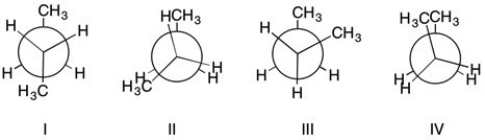
A) I<III<II<IV
B) IV<II<III<I
C) III<I<IV<II
D) II<IV<I<III
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide an acceptable name for (CH3CH2CH2)3CH.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the lowest energy chair conformation of cis-1,3-dimethylcyclohexane, how many axial positions are occupied by hydrogen atoms?
A) 2
B) 3
C) 4
D) 5
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many methylene groups are present in 2,4-dimethylhexane?
A) 0
B) 1
C) 2
D) 6
E) 8
Correct Answer

verified
Correct Answer
verified
Essay
Consider rotation about the C3-C4 bond of hexane, and draw the Newman projection for the most stable conformation.
Correct Answer

verified
Correct Answer
verified
Essay
In a Newman projection, siting down the C3-C4 bond, draw the structure below in its most stable conformation. 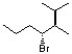
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide an acceptable name for [(CH3)3C]2CHCH3.
Correct Answer

verified
2,2,3,4,4-...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw the most stable conformation of cis-1,2-dimethylcyclohexane.
Correct Answer

verified
Correct Answer
verified
Short Answer
Name the alkane shown. [(CH3)2CH]2CHCH3
Correct Answer

verified
2,3,4-trim...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Convert the Newman structure below to the equivalent line-angle structure. 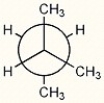
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding cyclobutane is correct?
A) The lowest energy conformation of cyclobutane is a planar one in which all of the bond angles is 90°.
B) The lowest energy conformation of cyclobutane is known as the chair conformation.
C) The lowest energy conformation is one in which the bond angles are slightly less than 90° even though this increases angle strain.
D) The lowest energy conformation is one in which the bond angles are greater than 90° so that angle strain is significantly reduced.
E) None of the above statements is correct.
Correct Answer

verified
Correct Answer
verified
Showing 121 - 134 of 134
Related Exams