A) I
B) II
C) III
D) Both I and II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the carbonyl compounds; (1) benzaldehyde,(2) acetophenone and (3) dicyclohexyl ketone,which compound has noa-hydrogens?
A) Benzaldehyde
B) Acetophenone
C) Acetone
D) All of these compounds contain a-hydrogens.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about Aldol reactions with either aldehydes or ketones is true?
A) Equilibrium favors the products with aldehydes; equilibrium favors the starting materials with ketones.
B) Equilibrium favors the starting materials with aldehydes; equilibrium favors the products with ketones.
C) Equilibrium favors the products with both aldehydes and ketones.
D) Equilibrium favors the starting materials with both aldehydes and ketones.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product of the following reaction? 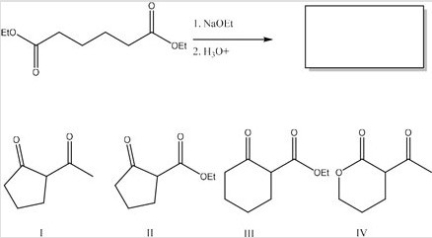
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following Claisen reaction? 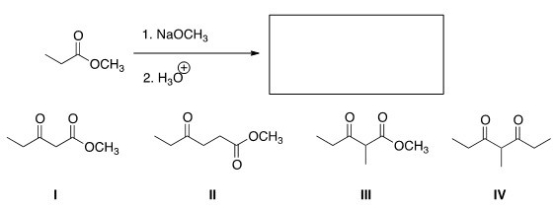
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name given to the Claisen reaction between two different esters?
A) Multiple Claisen reaction
B) Differential Claisen reaction
C) Crossed Claisen reaction
D) Versatile Claisen reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the general name for the reaction product formed in an aldol addition reaction?
A) a-Hydroxy carbonyl compound
B) b-Hydroxy carbonyl compound
C) g-Hydroxy carbonyl compound
D) a,b-Hydroxy carbonyl compound
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the correct order,what are the three general steps in the mechanism of a Claisen reaction?
A) Enolate formation,elimination,nucleophilic addition
B) Enolate formation,nucleophilic addition,elimination
C) Elimination,enolate formation,nucleophilic addition
D) Nucleophilic addition,enolate formation,elimination
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a Michael reaction,what is the name given to the a,b-unsaturated carbonyl component?
A) Michael donor
B) Michael enolate
C) Michael nucleophile
D) Michael acceptor
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the unsaturated carbonyl compound formed in the dehydration of the following b-hydroxy carbonyl compound? 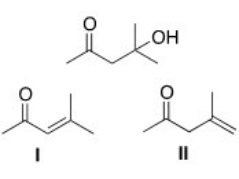
A) I
B) II
C) I and II
D) None of the choices
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is an intramolecular Claisen reaction called?
A) Michael reaction
B) Robinson reaction
C) Hoffman reaction
D) Dieckmann reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a Claisen condensation reaction is run using methyl propanoate as the reactant,NaOCH3 is the ideal base.Why is it important to use NaOCH3 and not NaOCH2CH3?
A) NaOCH3 is a stronger base than NaOCH2CH3,and this reaction requires a stronger base.
B) NaOCH3 is a weaker base than NaOCH2CH3,and this reaction requires a weaker base.
C) Transesterfication can occur when esters react,and this transesterfication would result in a mixture of products.
D) NaOCH3 is more soluble than NaOCH2CH3 in organic solvents,and this reaction requires a full equivalent of base to proceed.A full equivalent of NaOCH2CH3 would not dissolve,so the reaction would not proceed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following carbonyl compounds will undergo Aldol addition reactions when treated with aqueous sodium hydroxide? 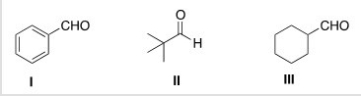
A) I
B) II
C) III
D) None of the choices
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product of the following reaction? 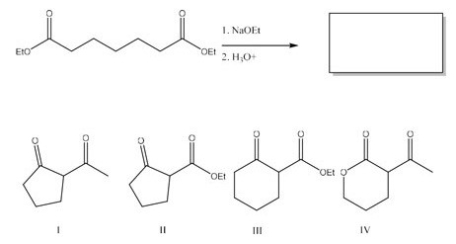
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Would this crossed Aldol reaction work well? Why or why not? 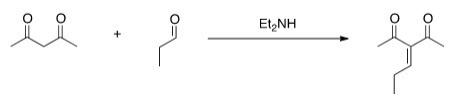
A) Yes,the diketone is significantly more acidic,so this enolate can be formed selectively.
B) Yes,the aldehyde is significantly more acidic,so this enolate can be formed selectively.
C) No,the aldehyde is significantly more acidic,so this enolate cannot be formed selectively.
D) No,the diketone is significantly more acidic,so this enolate cannot be formed selectively.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What reaction type is a Claisen reaction?
A) Electrophilic addition
B) Electrophilic substitution
C) Nucleophilic addition
D) Nucleophilic substitution
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the names for the two parts of the mechanism in a Robinson annulation?
A) Michael addition and intramolecular Claisen reaction
B) Michael addition and intramolecular aldol reaction
C) Claisen reaction and intramolecular aldol reaction
D) Aldol reaction and intramolecular Claisen reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is the Aldol reaction often called an Aldol condensation?
A) The initially formed b-hydroxy carbonyl compound loses a hydroxyl group.
B) The initially formed b-hydroxy carbonyl compound loses an oxygen atom.
C) The initially formed b-hydroxy carbonyl compound loses a hydrogen atom.
D) The initially formed b-hydroxy carbonyl compound loses water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the self-condensation of ethanal (acetaldehyde) ,shown below? 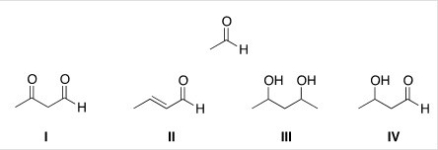
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When is a crossed Claisen reaction between two different esters synthetically useful?
A) When only one of the esters has a hydrogen atoms
B) When both esters have a hydrogen atoms
C) When only one of the esters has b hydrogen atoms
D) When both esters lack a hydrogen atoms
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 51
Related Exams