A) SN1
B) SN2
C) E1
D) E2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the most reactive substrate in an E1 reaction? 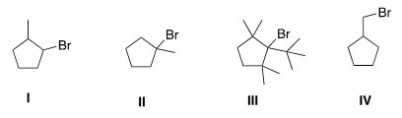
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 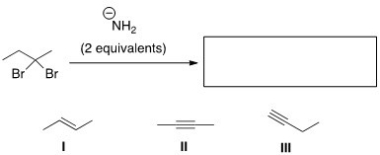
A) Only I
B) Only II
C) Only III
D) II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about an E1 mechanism is true?
A) The identity of the leaving group affects the rate of reaction.
B) The reaction follows second-order kinetics.
C) The reaction is slowest with tertiary substrates.
D) Polar aprotic solvents favor the E1 mechanism.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product and likely mechanism for the following reaction? 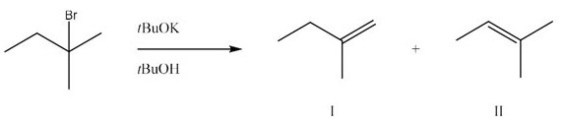
A) I,E1
B) I,E2
C) II,E1
D) II,E2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major elimination product obtained from the following reaction? 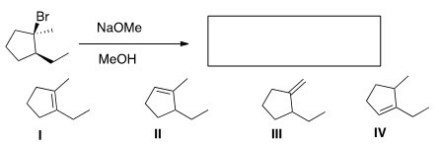
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the dihalide that can be used to prepare the alkyne below? 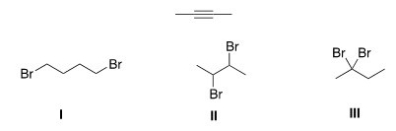
A) Only I
B) Only II
C) Only III
D) II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halide reacts the fastest in an E2 reaction? 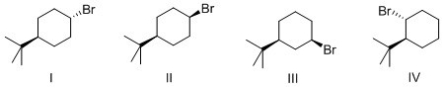
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following E2 reaction.What rate equation would be observed for this reaction? ![Consider the following E2 reaction.What rate equation would be observed for this reaction? A) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br] B) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br][KOC(CH<sub>3</sub>) <sub>3</sub>] C) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br][KOC(CH<sub>3</sub>) <sub>3</sub>]<sup>2</sup> D) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br]<sup>2</sup>[KOC(CH<sub>3</sub>) <sub>3</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f7e9_877f_a9af_332f0b95f3e4_TB7662_00.jpg)
A) Rate = k[CH3CH2CH2Br]
B) Rate = k[CH3CH2CH2Br][KOC(CH3) 3]
C) Rate = k[CH3CH2CH2Br][KOC(CH3) 3]2
D) Rate = k[CH3CH2CH2Br]2[KOC(CH3) 3]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many different E2 products can form from the dehydrohalogenation of 2-bromobutane?
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the elimination product(s) formed by treating the indicated alkyl halide with a base. 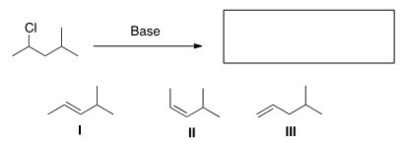
A) Only I
B) Only II
C) Only III
D) I,II,and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the least reactive substrate in an E2 reaction? 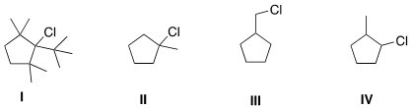
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Showing 41 - 52 of 52
Related Exams