A) +248.5
B) - 248.5
C) +485.4
D) +11.6
E) - 11.6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a reaction to be spontaneous under standard conditions at all temperatures, the signs of ΔH° and ΔS° must be _ _ and , respectively.
A) +, +
B) +, -
C) - , +
D) - , -
E) +, 0
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 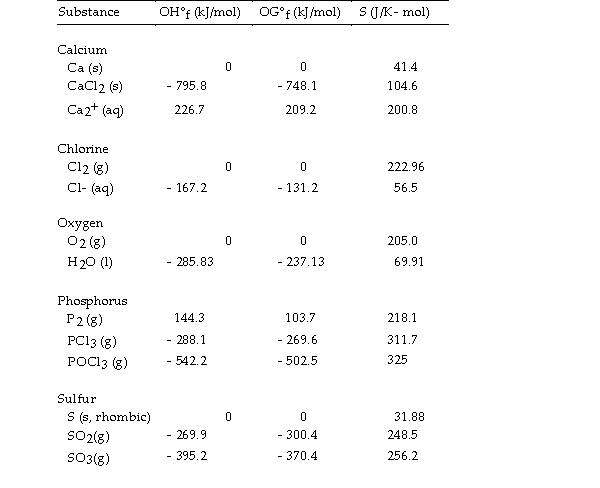 -The value of ΔH° for the formation of calcium chloride from its constituent elements,
-The value of ΔH° for the formation of calcium chloride from its constituent elements,  is kJ/mol.
is kJ/mol.
A) - 795.8
B) +795.8
C) +0.00
D) +397.9
E) - 397.9
Correct Answer

verified
Correct Answer
verified
Short Answer
Find the temperature above which a reaction with a OH of 123 .0 kJ/mol and a OS of 90.00 J/K·mol becomes spontaneous.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 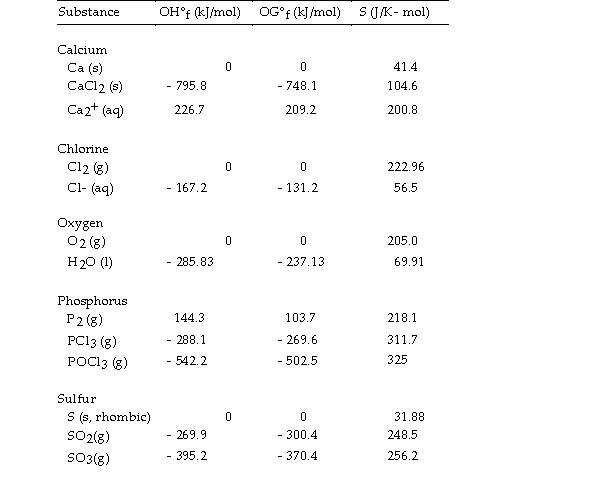 -The value of ΔS° for the formation of phosphorous trichloride from its constituent elements,
-The value of ΔS° for the formation of phosphorous trichloride from its constituent elements,  is J/K.
is J/K.
A) +129.4
B) +311.7
C) - 263.7
D) - 129.4
E) - 311.7
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, the entropy of gaseous _ is the largest at 25 °C and 1 atm.
A) C2H4
B) C2H2
C) C2H6
D) H2
E) CH4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 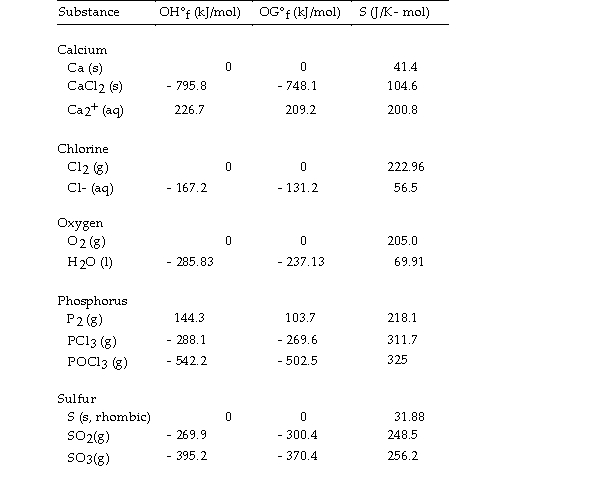 -The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements,
-The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements,  is kJ/mol.
is kJ/mol.
A) +790.4
B) +395.2
C) +105.1
D) - 395.2
E) - 790.4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 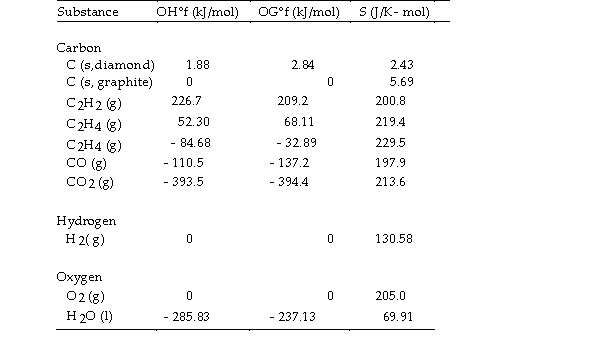 -The combustion of ethene in the presence of excess oxygen yields carbon dioxide and water:
-The combustion of ethene in the presence of excess oxygen yields carbon dioxide and water:
 The value of OSo for this reaction is J/K.
The value of OSo for this reaction is J/K.
A) - 347.6
B) +140.9
C) - 140.9
D) +347.6
E) - 267.4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 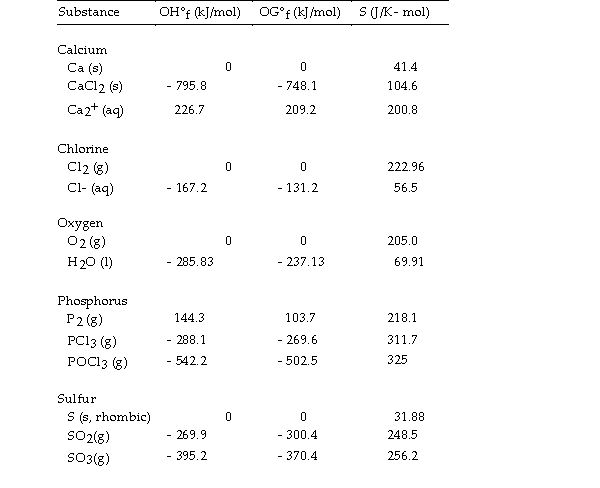 -The value of OS° for the formation of POCl3 from its constituent elements,
-The value of OS° for the formation of POCl3 from its constituent elements,  is J/K.
is J/K.
A) - 321
B) - 771
C) +321
D) +771
E) - 442
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 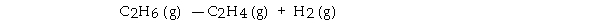 ΔH° is +137 kJ/mol and ΔS° is +120 J/K · mol. This reaction is .
ΔH° is +137 kJ/mol and ΔS° is +120 J/K · mol. This reaction is .
A) spontaneous only at high temperature
B) spontaneous at all temperatures
C) spontaneous only at low temperature
D) nonspontaneous at all temperatures
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A common name for methanol (CH3OH) is wood alcohol. The normal boiling point of methanol is 64.7 °C and the molar enthalpy of vaporization if 71.8 kJ/mol. The value of ΔS when 2.15 mol of CH3OH (l) vaporizes at 64.7 °C is J/K.
A) 5.21 × 107
B) 0.457
C) 457
D) 2.39
E) 2.39 × 103
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
-
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
-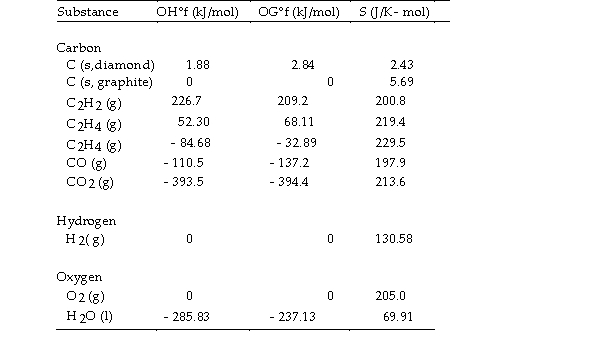 The value of ΔS° for the catalytic hydrogenation of acetylene
The value of ΔS° for the catalytic hydrogenation of acetylene  Is J/K.
Is J/K.
A) +550.8
B) +18.6
C) - 18.6
D) +112.0
E) - 112.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction that is spontaneous as written .
A) is very rapid
B) is very slow
C) will proceed without outside intervention
D) is also spontaneous in the reverse direction
E) has an equilibrium position that lies far to the left
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
-
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
-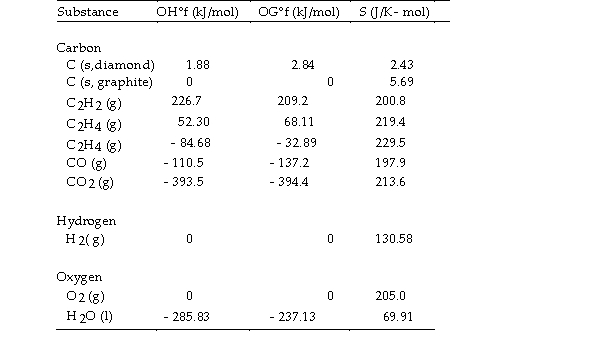 The value of ΔS° for the oxidation of carbon to carbon
The value of ΔS° for the oxidation of carbon to carbon  Is J/K. The combustion of carbon, as in charcoal briquettes, in the presence of abundant oxygen produces carbon dioxide.
Is J/K. The combustion of carbon, as in charcoal briquettes, in the presence of abundant oxygen produces carbon dioxide.
A) +2.9
B) - 2.9
C) - 205.0
D) +205.0
E) +424.3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, the entropy of is the largest.
A) HCl (g)
B) HCl (s)
C) HCl (l)
D) HBr (g)
E) HI (g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)  -The value of ΔS° for the formation of calcium chloride from its constituent elements,
-The value of ΔS° for the formation of calcium chloride from its constituent elements,  is J/K.
is J/K.
A) +104.6
B) - 104.6
C) +159.8
D) - 159.8
E) +369.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 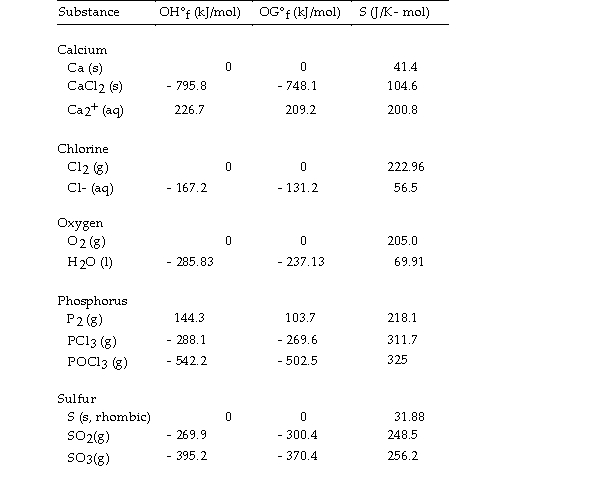 -The value of ΔG° at 25 °C for the decomposition of calcium chloride into its constituent elements,
-The value of ΔG° at 25 °C for the decomposition of calcium chloride into its constituent elements,  is kJ/mol.
is kJ/mol.
A) - 748.1
B) +748.1
C) +763.7
D) - 795.8
E) +795.8
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 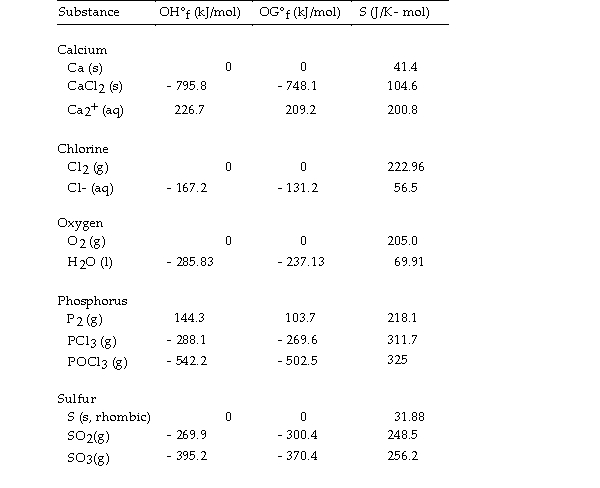 -The value of ΔG° at 25 °C for the formation of calcium chloride from its constituent elements,
-The value of ΔG° at 25 °C for the formation of calcium chloride from its constituent elements,  is kJ/mol.
is kJ/mol.
A) - 795.8
B) +748.1
C) +795.8
D) +763.7
E) - 748.1
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 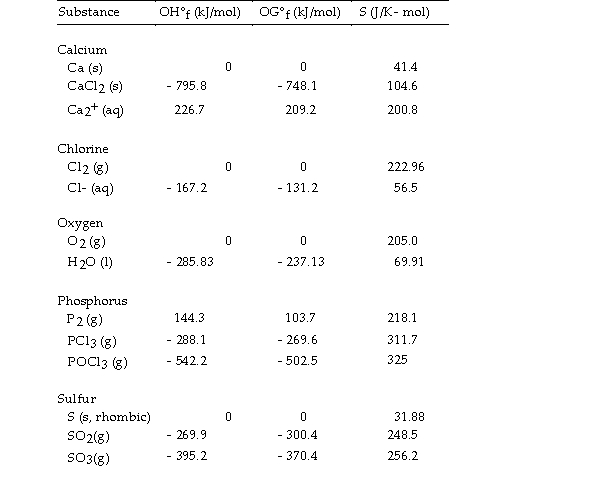 -The value of ΔS° for the reaction
2C (s, diamond) + O2 (g) - 2CO (g) is J/K.
-The value of ΔS° for the reaction
2C (s, diamond) + O2 (g) - 2CO (g) is J/K.
A) +9.5
B) - 195.7
C) - 9.5
D) - 185.9
E) +185.9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard Gibbs free energy of formation of is zero. (a) H2O (l) (b) Na (s) (c) H2 (g)
A) (a) only
B) (b) only
C) (c) only
D) (b) and (c)
E) (a) , (b) , and (c)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 101
Related Exams