A) 134
B) 0.347
C) 263
D) 395
E) 116
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of 0.65 mol of an ideal gas at 365 torr and 97 °C is _ L.
A) 0.054
B) 9.5
C) 41
D) 11
E) 2.4 × 10- 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of 50 mL of Cl2 gas with 50 mL of CH4 gas via the equation: Cl2 (g) + C2H4 (g) -C2H4Cl2 (g) Will produce a total of mL of products if pressure and temperature are kept constant.
A) 125
B) 150
C) 50
D) 25
E) 100
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 1.44- g sample of an unknown pure gas occupies a volume of 0.335 L at a pressure of 1.00 atm and a temperature of 100.0 °C. The unknown gas is _.
A) krypton
B) argon
C) helium
D) xenon
E) neon
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas vessel is attached to an open- end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below.
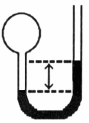 The difference in heights of the liquid in the two sides of the manometer is 32.3 cm when the atmospheric pressure is 765 mmHg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 cm when the atmospheric pressure is 765 mmHg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is atm.
A) 0.976
B) 1.08
C) 1.04
D) 1.01
E) 0.993
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The density of chlorine (Cl2) gas at 25 °C and 60. kPa is g/L)
A) 20
B) 4.9
C) 0.86
D) 0.58
E) 1.7
Correct Answer

verified
Correct Answer
verified
Short Answer
The rms speed of methane molecules at 45.0 °C is m/sec.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following gases would deviate the least from ideal gas behavior?
A) CH3Cl
B) F2
C) Ne
D) CO2
E) Kr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 667 torr. Use Boyle's law to calculate the pressure (torr) when the volume is reduced to 7.88 L at a constant temperature of 25.0 °C.
A) 526
B) 5.26 × 104
C) 1.11
D) 846
E) 0.118
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of a gas originally at 25 °C and 1.00 atm pressure in a 2.5 L container is allowed to expand until the pressure is 0.85 atm and the temperature is 15 °C. The final volume of the gas is L)
A) 0.38
B) 3.0
C) 2.1
D) 2.6
E) 2.8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Zinc reacts with aqueous sulfuric acid to form hydrogen gas:
 In an experiment, 225 mL of wet H2 is collected over water at 27 °C and a barometric pressure of 748 torr. How many grams of Zn have been consumed? The vapor pressure of water at 27 °C is
26) 74 torr.
In an experiment, 225 mL of wet H2 is collected over water at 27 °C and a barometric pressure of 748 torr. How many grams of Zn have been consumed? The vapor pressure of water at 27 °C is
26) 74 torr.
A) 567
B) 4.79 × 106
C) 4.31 × 105
D) 0.567
E) 431
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following gases in order of increasing average molecular speed at 25 °C. 
A) CO2 < N2 < O2 < He
B) He < N2 < O2 < CO2
C) CO2 < O2 < N2 < He
D) CO2 < He < N2 < O2
E) He < O2 < N2 < CO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calcium hydride (CaH2) reacts with water to form hydrogen gas:
 How many grams of CaH2 are needed to generate 48.0 L of H2 gas at a pressure of 0.888 atm and a temperature of 32 °C?
How many grams of CaH2 are needed to generate 48.0 L of H2 gas at a pressure of 0.888 atm and a temperature of 32 °C?
A) 50.7
B) 71.7
C) 143
D) 35.8
E) 0.851
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of oxygen gas (O2) was found to effuse at a rate equal to three times that of an unknown gas. The molecular weight of the unknown gas is g/mol.
A) 4
B) 10.7
C) 96
D) 288
E) 55
Correct Answer

verified
Correct Answer
verified
Short Answer
Given the equation 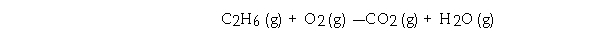 Determine the number of liters of O2 consumed at STP when 270.0 grams of C2H6 is burned.
Determine the number of liters of O2 consumed at STP when 270.0 grams of C2H6 is burned.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The density of ammonia gas in a 4.32 L container at 837 torr and 45.0 °C is g/L.
A) 3.86
B) 0.194
C) 0.717
D) 4.22 × 10- 2
E) 0.432
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A closed- end manometer was attached to a vessel containing argon. The difference in the mercury levels in the two arms of the manometer was 12.2 cm. Atmospheric pressure was 783 mmHg. The pressure of the argon in the container was mmHg.
A) 661
B) 122
C) 882
D) 771
E) 795
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The kinetic- molecular theory predicts that pressure rises as the temperature of a gas increases because _ .
A) the gas molecules collide less frequently with the wall
B) the gas molecules collide more frequently with the wall
C) the average kinetic energy of the gas molecules decreases
D) the gas molecules collide more energetically with the wall
E) both the gas molecules collide more frequently with the wall and the gas molecules collide more energetically with the wall
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ideal gas differs from a real gas in that the molecules of an ideal gas .
A) have no attraction for one another
B) have an average molecular mass
C) have a molecular weight of zero
D) have no kinetic energy
E) have appreciable molecular volumes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume (L) of NH3 gas at STP is produced by the complete reaction of 7.5 g of H2O according to the following reaction? 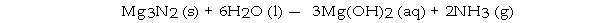
A) 3.1
B) 19
C) 28
D) 9.3
E) 0.32
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 146
Related Exams