A) stabilization
B) nuclear dissociation
C) radioactive decay
D) nuclear fusion
E) ionization
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which radioactive element is sometimes found in indoor air in such places as basements?
A) polonium
B) thallium
C) uranium
D) radon
E) plutonium
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Magnetic resonance imaging (MRI) depends on the presence of what type of particles in body tissue?
A) carbon atoms
B) hydrogen atoms
C) water molecules
D) radioactive isotopes
E) magnetic particles
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope 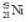 decays to
decays to 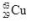 by emitting radiation.What type of radiation is emitted?
by emitting radiation.What type of radiation is emitted?
A) alpha
B) beta
C) gamma
D) positron
E) None of the choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What percentage of the initial amount of a radioactive isotope will remain after ten half-lives?
A) 0.50%
B) 0.78%
C) 0.36%
D) 0.11%
E) 0.098%
Correct Answer

verified
Correct Answer
verified
True/False
A radioactive sample will decay completely in two half-lives.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The term LD50 represents which of the following?
A) the dosage of toxic material needed to kill 50% of the exposed population in 30 days
B) the number of people killed by 1 mg of a toxic material in one day
C) the number of people killed by 1 mg of a toxic material in 30 days
D) the percentage of people that will die when exposed to 50 mg of a toxic substance
E) the amount of radiation absorbed by the body when standing 50 m from a radioactive substance
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From which of the following isotopes is carbon-14 formed by cosmic ray bombardment in the upper atmosphere?
A) Li-5
B) U-238
C) O-16
D) O-18
E) N-14
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What nuclear process is used in commercial nuclear power plants to produce energy?
A) fusion
B) fission
C) metastable induction
D) radiocarbon dating
E) positron emission tomography
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of radioactive decay is illustrated by the following nuclear equation? 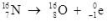
A) positron emission
B) alpha decay
C) beta decay
D) gamma production
E) helium emission
Correct Answer

verified
Correct Answer
verified
Essay
When the isotope 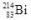 undergoes beta decay, what isotope is formed?
undergoes beta decay, what isotope is formed?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the process responsible for energy production in the sun?
A) chemical combustion
B) fissionoxidation-reduction
C) decomposition
D) fission
E) fusion
Correct Answer

verified
Correct Answer
verified
True/False
Gamma rays move at the speed of light.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement concerning nuclear reactions and chemical reactions is FALSE?
A) Nuclear reactions involve changes in the protons or neutrons in the nucleus of an atom.
B) Chemical reactions involve changes in the valence electrons of an atom.
C) Nuclear reactions usually result in a change in the identity of the radioactive isotope.
D) Nuclear reactions are capable of producing more energy than chemical reactions.
E) Chemical reactions always occur much faster than nuclear reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most widely used radioactive isotope in nuclear medicine?
A) technetium-99m
B) hydrogen-3
C) uranium-235
D) carbon-14
E) plutonium-244
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What does the symbol 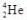 represent?
represent?
A) an alpha particle
B) a beta particle
C) a gamma ray
D) a positron
E) a deuteron
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which type of radiation emitted by radioactive nuclei has no mass and no charge?
A) alpha particle
B) beta particle
C) positron
D) gamma ray
E) All radiation types have a mass and charge.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of radioactive decay is illustrated by the following nuclear equation? 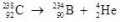
A) positron emission
B) alpha decay
C) beta decay
D) gamma production
E) helium emission
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope  decays to
decays to 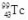 by emitting radiation.What type of radiation is emitted?
by emitting radiation.What type of radiation is emitted?
A) alpha particle
B) beta particle
C) positron
D) gamma ray
E) neutron
Correct Answer

verified
Correct Answer
verified
True/False
The mass number of an alpha particle is 4.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 82
Related Exams