A) The pressure of the gas on the outside of the balloon forces gas inside, making balloon A bigger.
B) The pressure on the outside of the balloon will shrink the gas particles, decreasing the volume of the balloon.
C) The pressure on the outside of the balloon will slow down the gas particles, making the balloon smaller.
D) The pressure outside the balloon will compress the air in the balloon, making the balloon smaller.
E) External pressure does not affect the volume of the balloon.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 1.0 atm, the air in a pilot's lungs occupies 0.50 L.The pilot ascends to an altitude where the pressure is 0.35 atm.If the pilot holds her breath during her ascent, what is the new volume of the air in the pilot's lungs?
A) 0.18 L
B) 0.70 L
C) 0.85 L
D) 1.4 L
E) 5.7 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At the grocery store, a bag of chips contains 0.10 L of air at 1.0 atm and 20 °C.The unopened bag of chips is taken on a camping trip in the mountains where the pressure is 0.90 atm and the temperature is 25 °C.What is the volume of air in the bag in the mountains?
A) 0.090 L
B) 0.11 L
C) 7.2 L
D) 0.14 L
E) 0.10 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements BEST describes pressure?
A) Pressure is energy applied to a given volume.
B) Pressure is work applied to a given area.
C) Pressure is work applied to a given volume.
D) Pressure is force applied to a given area.
E) Pressure is energy, work, or force applied to a substance.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement about vaporization and evaporation is FALSE?
A) Vaporization is an exothermic process, and evaporation is endothermic.
B) The heat required for evaporation can come from skin, resulting in cooling.
C) The heat of vaporization is equal in magnitude to the heat of condensation.
D) The heat of vaporization is the heat that is added per gram of liquid at its boiling point for vaporization.
E) Evaporation is a change of state from liquid to gas at a temperature below the boiling point.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond in acetone, shown below, is correctly labeled with a dipole arrow? 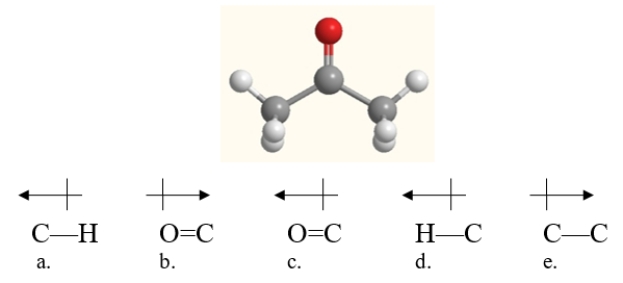
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement BEST describes how the kinetic molecular view of gases can be used to explain the effect of gas temperature on gas pressure?
A) Gas pressure and gas temperature are unrelated.
B) The effect of temperature on pressure depends upon the gas.
C) As temperature increases, the particles are damaged, slowing them down and decreasing the number of collisions against the beaker; therefore the pressure decreases.
D) As temperature increases, the particles are split apart so that there are more collisions against the beaker and the pressure increases.
E) As temperature increases, the speed of particles increases, so there are more collisions against the beaker and the pressure increases.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Imagine that you have a beaker of gas molecules as shown in the image below.If all of the gas in the beaker is transferred to a new beaker half the size of the original beaker, while maintaining the same temperature, which view BEST represents what the gas would look like? 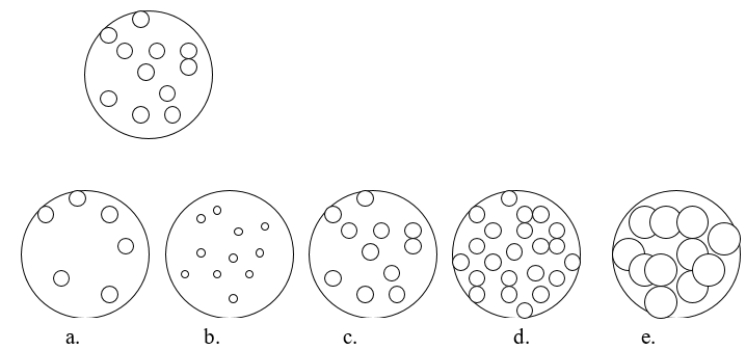
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
To accomplish the change of phase shown in the diagram below, you could 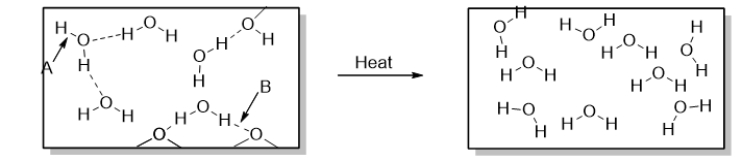
A) drink a glass of water.
B) put water in the freezer.
C) boil water.
D) let an ice cube melt in a warm glass of water.
E) let an ice cube melt in the sink.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following changes is NOT correctly labeled as a chemical reaction or a change of state?
A) rust forming on metal, a chemical reaction
B) steam coming off of a cup of coffee, a change of state
C) an ice cube melting in a drink, a change of state
D) the combustion of gasoline to give carbon dioxide and water, a chemical reaction
E) bubbles forming in a just-opened can of soda, a chemical reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A narrow tube on a road bike should be inflated to about 100 psi.What is this pressure in atmospheres?
A) 0.1 atm
B) 0.5 atm
C) 7 atm
D) 1,000 atm
E) 2 × 105 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atmospheric pressure at sea level is ________.At elevations higher than sea level, atmospheric pressure is_________.
A) less than 1 atm; 1 atm
B) 1 atm; less than 1 atm
C) greater than 1 atm; 1 atm
D) 1 atm; greater than 1 atm
E) 1 atm; impossible to predict
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What happens when a large volume of gas is compressed to a smaller volume?
A) There are more gas particles in the same volume.
B) The particles shrink.
C) The gas particles would expand in comparison to the size of the beaker.
D) Compressing the gas would likely result in gas particles lost to the atmosphere.
E) Compressing the gas is just a physical change and so should not affect how the gas particles look.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
This diagram shows 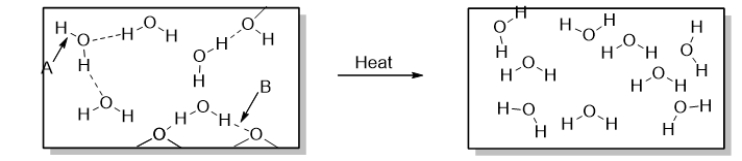
A) the decomposition of water.
B) the combustion of water.
C) solid water (ice) changing to liquid water (melting) .
D) liquid water changing to water vapor (boiling) .
E) water vapor changing to liquid water (condensation) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is water (H2O) a liquid at room temperature whereas methane (CH4) is a gas?
A) Water forms hydrogen bonds, and methane only forms dispersion forces.
B) Water forms dispersion forces, and methane forms hydrogen bonds.
C) Water has a higher molecular weight than methane.
D) Methane has a higher molecular weight than water.
E) Methane has more atoms than water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The two beakers below each have added to them the same amount of heat energy.Which statement BEST describes what would happen to the temperatures of the two beakers? 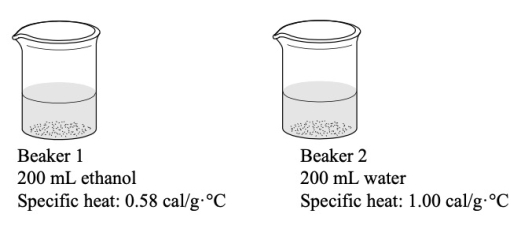
A) The temperature of the two beakers will remain the same.
B) The temperatures of the two beakers will increase by the same amount.
C) The temperature of beaker 1 will increase more than that of beaker 2.
D) The temperature of beaker 2 will increase more than that of beaker 1.
E) It is not possible to predict how the temperature of the beakers will change.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A scuba diver dives down to 15 m, where the pressure is 2.5 atm.The scuba diver then inhales 500.mL of air and holds his breath while ascending to the water surface, where the pressure is 1 atm.What is the volume of the air in the diver's lungs at the surface? Assume that T and n are constant.
A) 0.0050 mL
B) 0.20 mL
C) 5.0 mL
D) 200 mL
E) 1,250 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement BEST describes why a higher concentration of carbon dioxide is present in exhaled breath compared to inhaled breath?
A) Actually, a higher concentration of carbon dioxide is present in inhaled breath.
B) Once the oxygen is used up in an inhalation, a larger percentage is carbon dioxide.
C) The oxygen in inhaled air reacts in the lungs to make carbon dioxide.
D) The concentration of carbon dioxide in the tissue is lower than that in exhaled air.
E) Carbon dioxide is produced during cellular respiration and then carried by the blood to the lungs to be exhaled.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetone, shown here, is a common solvent and component of fingernail polish remover.Which of the following Lewis structures is acetone? 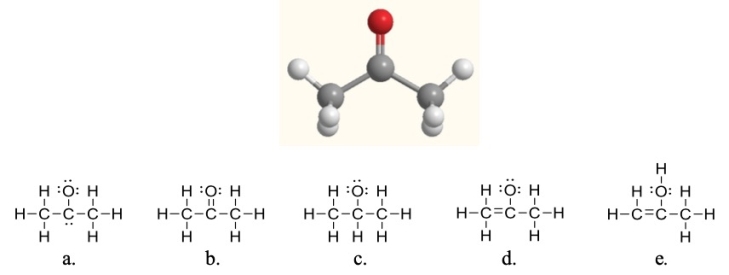
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to Avogadro's law, 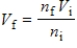 .What is the meaning of this law?
.What is the meaning of this law?
A) Adding moles of gas decreases the volume of the gas.
B) Adding moles of gas increases the volume of the gas.
C) The volume is inversely proportional to the moles of air.
D) Gas particles shrink when they are crowded.
E) Gas particles expand when they are crowded.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 99
Related Exams