A) oxygen
B) carbon dioxide
C) exhaled water
D) body heat
E) food intake
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is a biochemical pathway different than a biochemical reaction?
A) Actually, they are identical.
B) Only pathways occur in cells not reactions.
C) Only reactions occur in cells not pathways.
D) Reactions are catabolic, and pathways are anabolic.
E) A pathway is a specific sequence of reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following processes are anabolic?
A) muscle building as a result of steroid use
B) releasing energy from food during the citric acid cycle
C) weight loss as a result of burning fat
D) transferring bond energy from food to ATP
E) All of the above are anabolic processes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In order for a reaction to occur, reactant molecules must attain a certain amount of energy called _______.
A) ΔH
B) energy
C) entropy
D) activation energy
E) Any of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements describe how oxygen intake relates to caloric requirements? I.Oxygen reacts with food to produce energy. II) The larger the oxygen intake, the higher the energy output. III) Oxygen is a product of food metabolism.
A) All of these statements describe the relationship between oxygen intake and caloric requirements.
B) I and II describe the relationship between oxygen intake and caloric requirements.
C) II and III describe the relationship between oxygen intake and caloric requirements.
D) I and III describe the relationship between oxygen intake and caloric requirements.
E) Only II describes the relationship between oxygen intake and caloric requirements.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are biological catalysts called?
A) proteins
B) nucleic acids
C) enzymes
D) carbohydrates
E) lipids
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One serving of animal crackers (30 g) contains 4.5 g of fat, 22 g of carbohydrates, and 2 g of proteins.Which of the following equations would you use to determine the number of calories in one serving of animal crackers?
A) 4.5 g × 4 Cal/g + 22 g × 9 Cal/g + 2 g × 4 Cal/g
B) 4.5 g × 9 Cal/g + 22 g × 4 Cal/g + 2 g × 4 Cal/g
C) 4.5 g × 4 Cal/g + 22 g × 4 Cal/g + 2 g × 4 Cal/g
D) 4.5 g × 9 Cal/g + 22 g × 9 Cal/g + 2 g × 4 Cal/g
E) 4.5 g × 4 Cal/g + 22 g × 4 Cal/g + 2 g × 9 Cal/g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How would this diagram be different if the reaction was exothermic? 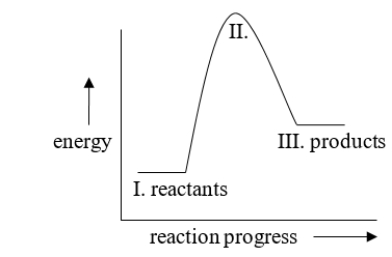
A) III would be lower than I
B) No difference; this reaction is already exothermic.
C) II would be lower than III.
D) II would be lower, between I and III.
E) II would be lower than I.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following biological molecules are the major nutrients that make up the food that we eat? I. Proteins II.Nucleic acids III.Steroids IV.Fats V.Carbohydrates
A) All of these are major nutrients.
B) I, II, IV, and V
C) I and V
D) III, IV, and V
E) I, IV, and V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is the reaction that occurs in a calorimeter different from human metabolism?
A) One is a combustion reaction, and the other is not.
B) One occurs in many steps, and the other does not.
C) One is exothermic, and the other is endothermic.
D) One releases carbon dioxide, and the other does not.
E) These reactions are exactly the same.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction between acetic acid (CH3COOH) and methanol (CH3OH) is at equilibrium.Select the change that would result in a shift to the right. CH3COOH + CH3OH ⇌ CH3COOCH3 + H2O
A) adding more CH3COOH
B) adding more H2O
C) adding more CH3COOCH3
D) removing CH3OH
E) Both B and C are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which part of the following energy diagram is changed when a catalyst is added, and how is it changed? 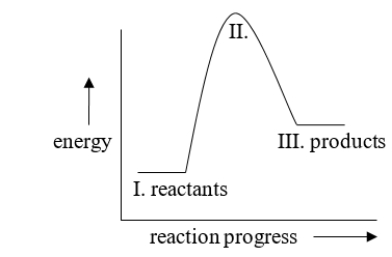
A) I is higher.
B) II is higher.
C) III is higher.
D) I is lower.
E) II is lower.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Bond breaking is always endothermic and yet catabolism, the breakdown of nutrients, is an overall exothermic process.How can you reconcile these two statements?
A) Catabolism is an exception to the rule.
B) Bond breaking is not really always endothermic.
C) Endothermic bond breaking is only seen in the laboratory.
D) No one really understands this.
E) Catabolism is the sum of many reactions, including many bond-making reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An exothermic reaction is one that:
A) has a positive ΔH.
B) has no ΔH.
C) absorbs heat.
D) has products higher in energy than reactants.
E) has a negative ΔH.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement describes reaction rate?
A) Reaction rate is how fast a reaction proceeds.
B) Reaction rate is measured by the disappearance of reactants over time.
C) Reaction rate is measured by the appearance of products formed over time.
D) Reaction rate is determined, in part, by activation energy.
E) All of the above statements describe reaction rate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement best describes the relationship between the heat of reaction and reaction rate?
A) There is no readily predictable relationship between ΔH and reaction rate.
B) Exothermic reactions are always slow.
C) Endothermic reactions are always slow.
D) The heat of reaction is always larger than reaction rate.
E) The relationship depends on whether the reaction is endothermic or exothermic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the false statement regarding bond energy.
A) Bonds with higher bond energies are stronger.
B) Stronger bonds have lower potential energy.
C) A single bond is stronger than a double bond.
D) A triple bond is stronger than a double bond.
E) Compounds with weaker bonds are more reactive.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ΔH for the reaction described by the energy diagram below is 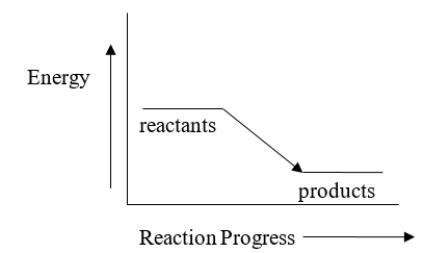
A) very large.
B) greater than zero but not necessarily very large.
C) exactly zero.
D) less than zero.
E) It is not possible to predict anything about the ΔH of this equation.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An endothermic reaction is one that
A) releases heat.
B) has no heat of reaction.
C) absorbs heat.
D) has reactants higher in energy than products.
E) has a negative heat of reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction occurs between the large black spheres and the small grey spheres.Each of the boxes below represents different reaction conditions for that reaction.Which box contains the reaction conditions that leads to the fastest reaction? 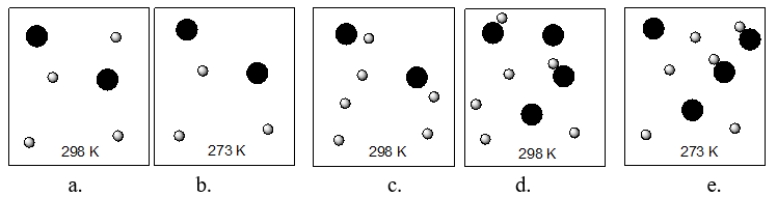
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 87
Related Exams