A) one
B) two
C) three
D) four
E) five
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen trichloride, once used as a bleaching agent, causes neurological disorder and was banned in 1949.Which of the following is a correct Lewis structure for nitrogen trichloride (NCl3) ? 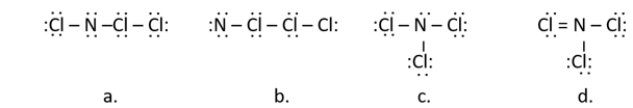
A) structure a
B) structure b
C) structure c
D) structure d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the electron geometry of the carbon in chloroform (CHCl3) ?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the strongest type of intermolecular interaction attracting molecules of propane together? 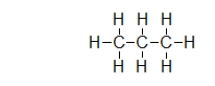
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element is the LEAST electronegative?
A) potassium
B) hydrogen
C) fluorine
D) carbon
E) oxygen
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Formaldehyde is used as a preservative and disinfectant.It has the structure shown below.What is the strongest type of intermolecular interaction attracting molecules of formaldehyde together? 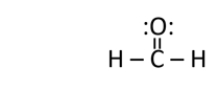
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of the atoms bonded to the nitrogen atom indicated with arrow II? 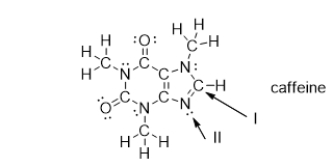
A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) linear
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom in a molecule has one lone pair and three atoms bonded to it.What is the molecular geometry of this atom?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Does formaldehyde have a permanent dipole? 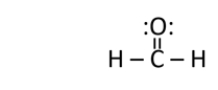
A) Yes.The carbon is partially negative, and the oxygen is partially positive.
B) Yes.The carbon is partially negative, and the hydrogen is partially positive.
C) Yes.The carbon is partially positive, and the oxygen is partially negative.
D) Yes.The carbon is partially positive, and the hydrogen is partially negative.
E) No.Formaldehyde only has a temporary dipole.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom in a molecule has a trigonal planar molecular geometry and a formula of MX3.What is the angle between the atoms in this molecule?
A) 360º
B) 180º
C) 120º
D) 109.5º
E) 90º
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules contains polar bonds but is nonpolar overall?
A) Cl2
B) CF4
C) H2O
D) N2
E) NH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ethylene is used as a starting material in making plastics.It has a molecular formula of C2H4.Which of the following structures is the correct Lewis structure for ethylene? 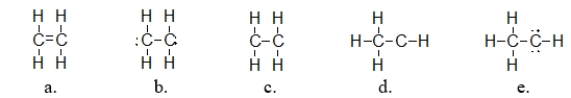
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following steps is done first to determine the molecular geometry of dichloromethane (CH2Cl2) ?
A) Calculate the molecular weight.
B) Look up the bond angle of a H-C-H bond.
C) Determine the electron geometry.
D) Construct the Lewis structure.
E) The molecular geometry can be determined without doing any prior steps.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of the carbon in dichloromethane (CH2Cl2) ?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which type(s) of molecular model illustrate the three-dimensional shape of a molecule?
A) ball-and-stick
B) Lewis dot structure
C) space-filling
D) Lewis dot and space-filling
E) ball-and-stick and space-filling
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom in a molecule has two lone pairs and two atoms bonded to it.What is the molecular geometry of this atom?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the molecules below does NOT have a permanent dipole? 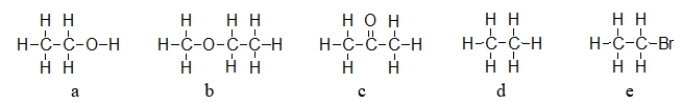
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following interactions is the strongest?
A) dipole-dipole forces
B) hydrogen bonding forces
C) dispersion forces
D) covalent bond
E) All of the above interactions have the same strength.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many nonbonding pairs are on the carbon in dichloromethane (CH2Cl2) ?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In molecules with permanent dipoles, _______ are the attraction of the positive end of a dipole on one molecule with the negative end of the dipole on another molecule.
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 86
Related Exams