A) I only
B) I and II
C) II and III
D) III, IV, and V
E) all of these
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which reactant and product would balance the following nuclear reaction equation? Reactant + 12C 11B + product
A) reactant = -, product = 1H
B) reactant = +, product = 1n
C) reactant = -, product = 1n
D) reactant = +, product =
E) reactant = 1H, product =
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The activity of a sample of gas obtained from a basement containing radon-222 was found to be 8 pCi/L. This isotope has a half-life of 3.8 days. If no additional radon-222 entered the basement, how long would it take for the activity to decline to 1 pCi/L?
A) about 4 days
B) a bit more than 10 days
C) about 1 day
D) a bit less than 10 days
E) about 20 days
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What quantity of energy would be produced as one atom of plutonium-238 undergoes alpha decay? The nuclide mass of 238Pu is 238.0495 amu (3.953 *10-22 g) , and the nuclide mass of uranium-234 is 234.0409 amu (3.886 *10-22 g) . Alpha particle mass is 6.64465 *10-24g. The speed of light is 2.998 × 10? m/s.
A) 6.0 *10-7 J
B) 5.0 * 10-12 J
C) 7.0 *10-10 J
D) 2.6 *10-8 J
E) 1.1 *10-12 J
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
A half-life is ________
A) the life that a nuclear chemist leads.
B) half of the lifetime of an unstable nucleus.
C) the time for one-half of the unstable nuclei to decay.
D) constantly changing.
E) independent of the rate constant for decay.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phosphorus-32 is a radioactive isotope used as a tracer in the liver. How much phosphorus-32 was originally used if there is only 3.50 mg left in a sample after 288 h? (The half-life of phosphorus-32 is 14.3 days.)
A) 1.96 mg
B) 6.26 mg
C) 4.17 mg
D) 7.00 mg
E) 17.9 mg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a nitrogen-14 nuclide captures an alpha particle, a proton is produced along with ________
A) neutrons.
B) boron-10.
C) oxygen-17.
D) fluorine-18.
E) carbon-17.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following units is used to quantify the biological effects of radiation?
A) curies
B) sieverts
C) rads
D) becquerels
E) joules
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Electricity is produced from nuclear reactions by ________
A) capturing the electrons that are emitted.
B) accelerating electrons with rapidly moving protons from the nuclear reaction.
C) a process still not understood by scientists.
D) using the energy to make steam to turn turbines.
E) using the energy to accelerate electrons in wires.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Low-level nuclear waste from medical facilities generally requires only short-term storage before disposal because ________
A) the concentrations of radioactive isotopes used in medicine are very low.
B) the radioactive isotopes are degraded by the medical procedures.
C) the radioactive isotopes used in medicine have short half-lives.
D) the radioactive isotopes used in medicine are selected because they are naturally safe.
E) there is little government regulation regarding the disposal of medical waste.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radiation seed therapy is a common method for treating some prostate cancers and brain tumors. Palladium-103, with a half-life of 17 days, is one isotope used for this treatment. How long does it take for the activity of palladium-103 to decrease to 1.0% of its initial value and essentially cease to be effective?
A) 3.7 mo
B) 13 mo
C) 2.1 mo
D) 7.4 mo
E) 4.2 mo
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which type of radiation does the most tissue damage, but only when the emitter is internally ingested?
A) alpha
B) beta
C) gamma
D) neutron
E) positron
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What quantity of energy would be produced as 1.00 kg of plutonium-238 undergoes alpha decay? The atomic mass of 238Pu is 238.0495 amu (3.953*10-22 g) , and the atomic mass of uranium-234 is 234.0409 amu (3.886* 10-22 g) . Alpha particle mass is 6.64465 * 10-24g. The speed of light is 2.998 × 10? m/s.
A) 4.4 * 1013 J
B) 3.5 * 1011 J
C) 6.2 *10-10 J
D) 1.3 * 1013 J
E) 2.7 *1012 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 10.00 g sample of wood from an archaeological site produced 3,072 particles in a 10-hour measurement owing to the presence of carbon-14, while a 10.00 g sample of new wood produced 9,216 particles in the same period of time. The half-life of carbon-14 is 5,730 years. How old is the wood from the archaeological site?
A) 5,730 years
B) 2,865 years
C) 4,040 years
D) 9,080 years
E) The correct answer differs by more than 100 years from the values given in A-D.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is a nuclear power plant not in danger of producing a nuclear explosion?
A) Control rods are in place that absorb all the neutrons.
B) The uranium-235 fuel is not pure enough.
C) The uranium-235 fuel is kept cool by a cooling system.
D) The uranium-235 fuel is not able to produce an explosive chain reaction.
E) Nuclear explosions can only occur through fusion, not fission.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which process is not part of the pathway that produces 4He nuclides in our sun?
A) (1p + 1p 2H + )
B) (2H + 1p 3He)
C) (2 3He 4He + 2 1p
D) (2 3He 10 + 2 1p)
E) (1p + 1n 2H)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In 1989, two prominent electrochemists, Stanley Pons and Martin Fleischmann, erroneously proposed that they had observed cold fusion, which was the term used to describe anomalously high energy generation associated with the electrolysis of deuterium oxide (D2O or heavy water) . Calculate the energy released when deuterium nuclides (2.013553 u) combine to produce a helium nuclide (4.001506 u) . (u = atomic mass unit)
A) 4 * 10-6 J
B) 4 * 10-8 J
C) 4*10-10 J
D) 4 *10-12 J
E) 4 *10-14 J
Correct Answer

verified
Correct Answer
verified
Essay
Bombarding atoms with alpha particles is a common way to synthesize new nuclides. Ernest Rutherford produced oxygen-17 from nitrogen-14 this way in 1919. Write a balanced reaction equation for this synthesis.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radon-222 has a half-life of 3.8 days. A sample from a basement in Colorado was analyzed 5) 0 days after it was collected and found to have an activity of 0.17 Bq. What was the original activity of this sample?
A) 0.22 Bq
B) 0.32 Bq
C) 0.42 Bq
D) 0.62 Bq
E) 0.52 Bq
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which graph below illustrates decay? 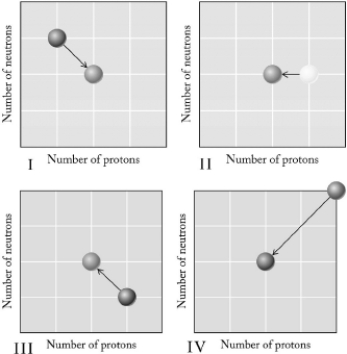
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 144
Related Exams