Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following standard reduction potentials. Which reaction A-D will proceed spontaneously as written from left to right?
A) 2Cr3+(aq) + 3Cd(s) 2Cr(s) + 3Cd2+(aq)
B) 2Cr3+(aq) + 3Ni(s) 2Cr(s) + 3Ni2+(aq)
C) Cd2+(aq) + Ni(s) Cd(s) + Ni2+(aq)
D) Cd(s) + Ni2+(aq) Cd2+(aq) + Ni(s)
E) None of these will proceed spontaneously.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When aluminum metal is obtained from aluminum oxide (Al2O3) , ________ moles of electrons must be transferred for each mole of aluminum oxide processed.
A) 2
B) 3
C) 4
D) 6
E) 9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A voltaic cell is constructed based on the oxidation of zinc metal and the reduction of silver cations. Solutions of silver nitrate and zinc nitrate also were used. Locate the silver nitrate on the diagram. 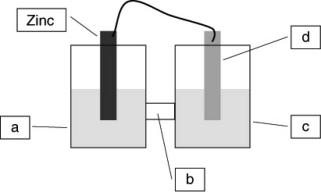
A) a
B) b
C) c
D) d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement about a voltaic cell is not correct?
A) Electrons are produced as a product at the cathode.
B) Reduction occurs at the cathode.
C) Usually the cathode is a metal strip.
D) In the external circuit, electrons flow toward the cathode.
E) Chemical species can have their oxidation number decreased at the cathode.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement about corrosion is not correct?
A) Corrosion is promoted by the presence of water.
B) Corrosion is promoted by the presence of electrolytes.
C) Corrosion is promoted by contact between dissimilar metals.
D) Surface oxidation protects metals such as iron, stainless steel, and aluminum from corrosion.
E) Cathodic protection prevents corrosion of an object by attaching it to a metal that serves as the cathode of the resulting electrochemical cell.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following standard reduction potentials. The Mg/Mg2+ half-reaction can be paired with the other two to produce voltaic cells because ________
A) Mg is a powerful oxidizing agent.
B) Mg is a powerful reducing agent.
C) Mg2+ is a powerful reducing agent.
D) Mg2+ is a powerful oxidizing agent.
E) Zn and Cu+ are readily oxidized.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The oxidation of hydrogen by oxygen is one of the most-used reactions in fuel-cell technology. The overall reaction, which is given below, has a G° value of -237 kJ/mol. What is the standard cell potential for this fuel cell? H2(g) + O2(g) H2O(l)
A) 2.46 V
B) 4.91 V
C) 1.23 V
D) 3.05 V
E) 1.50 V
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the change in free energy for a 1.50-V alkaline battery in which 2 moles of electrons are transferred from the anode to the cathode?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest oxidizing agent. Standard Reduction Potentials (volts) in Aqueous Solution
A) Pb4+
B) Pb2+
C) K+
D) K
E) Al
Correct Answer

verified
Correct Answer
verified
Short Answer
Chromium often is electroplated on other metals and even plastics to produce a shiny metallic appearance. How many grams of chromium would plate out from a solution of Cr(NO3)3 when 10 amps of current are passed through the electrolytic cell for 5.36 hours?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Neuron cells generate electrical signals by concentration gradients across membranes. Assuming a potassium ion concentration of 0.003 M inside the cell, and a concentration of 0.135 M outside the cell, what is the electrical potential across the cell membrane? Body temperature is 310 K. The sign identifies the change in the electrical potential across the membrane and which way the ions flow.
A) +204 mV
B) +102 mV
C) +10.0 mV
D) -204 mV
E) -136 mV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The energy supplied by a battery can be determined by ________
A) multiplying amperes supplied by seconds.
B) dividing amperes supplied by seconds.
C) multiplying coulombs supplied by volts.
D) multiplying coulombs supplied by seconds.
E) dividing coulombs supplied by seconds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the voltaic cell based on the following reaction: Ma(s) + Mb+(aq) Ma+(aq) + Mb(s) Two students are assigned to measure the standard cell potential. Yvonne claims that both solutions of ions have to be at exactly 1.00 M concentration, but Zelda is sure that the measurement will be the same if the concentrations of the two solutions are equal, but not necessarily 1.00 M. What do you think? (The temperature is controlled at 25°C, so that's not an issue.)
A) Yvonne is right because by definition standard cell potentials must be measured at concentrations of 1.00 M.
B) Yvonne is right because she understands the Nernst equation and what it describes.
C) Zelda is right because she understands the Nernst equation and what it describes.
D) Zelda is right because cell potentials do not depend on the concentration.
E) Both are right because cell potentials do not depend on the concentration.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The unit of current, ampere (A) , is defined as ________
A) 1 C.
B) 1 C/s.
C) 1 mol of electrons.
D) 1 mol of electrons per second.
E) 96,500 C/s.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A NiMH battery uses ________ as the reducing agent.
A) nickel
B) a metal hydride
C) hydroxide
D) hydronium ion
E) nickel oxide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How do fuel cells differ from traditional electrochemical cells (batteries) ?
A) The supply of reactants is continually renewed.
B) The cell potentials generally are lower.
C) The cell potentials generally are higher.
D) Fuel cells are closed systems; batteries are open systems.
E) Fuel cells do not involve oxidation-reduction reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction occurs in a new battery called the super iron battery. In this reaction, ________ is oxidized. 2K2FeO4(aq) + 3Zn(s) Fe2O3(s) + ZnO(s) + 2K2ZnO2(aq)
A) Fe in K2FeO4
B) Zn metal
C) Fe in Fe2O3
D) Zn in ZnO
E) Zn in K2ZnO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The spontaneous redox reaction in a voltaic cell has ________
A) a negative value of Ecell and a negative value of G.
B) a positive value of Ecell and a positive value of G.
C) a negative value of Ecell and a positive value of G.
D) a positive value of Ecell and a negative value of G.
E) a positive value of Ecell and a value of zero for G.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement regarding voltaic cells is not correct?
A) Reduction occurs at the cathode.
B) Anions move through the barrier or bridge toward the electrode where oxidation is occurring.
C) The electrode where reduction is occurring is represented by a positive sign.
D) Electrons flow in the external circuit from the cathode to the anode.
E) Electrons flow in the external circuit toward the electrode represented by a positive sign.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 143
Related Exams