A) Heat energy breaks the intermolecular forces holding molecules of water together.
B) Heat energy is released from intermolecular forces as the molecules of water break apart.
C) Heat energy breaks the covalent bonds holding molecules of water together.
D) Heat energy is released from covalent bonds as the molecules of water break apart.
E) Heat energy is not involved in this change.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why must a SCUBA diver ascend slowly from a dive?
A) to conserve air
B) to avoid the formation of nitrogen bubbles in the bloodstream
C) to avoid breathlessness
D) to allow time to change air tanks
E) There is no reason to ascend slowly from a dive.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A narrow tube on a road bike should be inflated to about 100 psi. What is this pressure in atmospheres?
A) 0.1 atm
B) 0.5 atm
C) 7 atm
D) 1000 atm
E) 2 105 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which process requires more energy per gram: melting ice or boiling water?
A) They both require the same amount of energy because both processes involve breaking intermolecular forces.
B) Melting ice takes more energy because more intermolecular forces are broken.
C) Boiling water takes more energy because more intermolecular forces are broken.
D) Melting ice takes more energy because it occurs at 0C instead of 100C.
E) The energy of these processes has never been compared.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Henry's law is P = kC. Which statement best describes the meaning of this law?
A) As the pressure above a gas in solution decreases, the concentration of gas in solution increases.
B) As the pressure above a gas in solution increases, the concentration of gas in solution also increases.
C) As the pressure above a gas in solution increases, the concentration of gas in solution decreases.
D) As the concentration of a gas in solution increases, Henry's constant increases.
E) As the concentration of a gas in solution decreases, Henry's constant decreases.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An oxygen tank has a pressure of 12 atm at 295 K. The maximum pressure that the tank can hold is 25 atm. What is the maximum temperature at which the tank can be stored?
A) 610 K
B) 590 K
C) 350 K
D) 140 K
E) 0.98 K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atmospheric pressure in Denver is 0.85 atm. What is the atmospheric pressure in torr?
A) 0.0011 torr
B) 890 torr
C) 650 torr
D) 12 torr
E) 0.058 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Balloon A is placed into a container that has a higher pressure than atmospheric pressure. Which balloon would you predict to be the new size of balloon A? 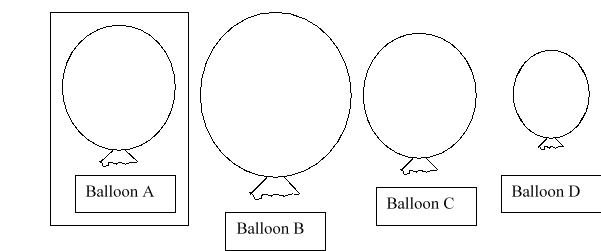
A) balloon B
B) balloon C
C) balloon D
D) either balloon B or D
E) either balloon B or C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You are riding your bike and run over a thorn, puncturing your tire. The air in the tire is released, resulting in a flat. What is the relationship between the volume (Vi) and moles of air (ni) in the inflated tire and the volume (Vf) and moles of air in the flat tire (nf) ?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) There is no relationship between the moles of air and the volume of air in the tire.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following statements is the gas variable correctly described?
A) Temperature is a measure of the space occupied by a gas.
B) Volume increases as the collisions of gas particles with the walls of the container increases.
C) Temperature is related to the average speed of gas particles.
D) The number of moles of a gas decreases as the volume of the gas increases.
E) When gas particles move faster, the pressure of the gas must increase.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to Avogadro's Law, What is the meaning of this law?
A) Adding moles of gas decreases the volume of the gas.
B) Adding moles of gas increases the volume of the gas.
C) The volume is inversely proportional to the moles of air.
D) Gas particles shrink when they are crowded.
E) Gas particles expand when they are crowded.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What happens to acetone when it is boiled? 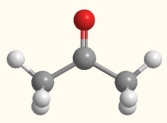
A) More intermolecular forces are formed.
B) The nonpolar covalent bonds are broken.
C) The polar covalent bonds are broken.
D) All bonds in the molecule are broken.
E) The intermolecular forces are broken.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You drive your car from Salt Lake City up into the mountains to go skiing. It is a nice day in Salt Lake, with a temperature of 16C and an atmospheric pressure of 0.85 atm. Up in the mountains, it is freezing at 1.0C and an atmospheric pressure of 0.70 atm. If the volume of air in your tires is 12 L when you leave the city, what is it in the mountains?
A) "0.91 L"
B) "9.3 L"
C) "11 L"
D) "13 L"
E) "14 L"
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does the pressure of a gas change when it is compressed?
A) It will not change because there is no relationship between volume and pressure.
B) It will increase because P1V1 = P2V2.
C) It will decrease because P1V1 = P2V2.
D) It will increase because P1/V1 = P2/V2.
E) It will decrease because P1/V1 = P2/V2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are ways to increase the pressure of a gas?
A) heating the gas
B) decreasing the volume of the gas by placing it into a smaller container
C) adding more gas molecules to the container
D) compressing the gas
E) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What occurs over the course of this physical change illustrated below? 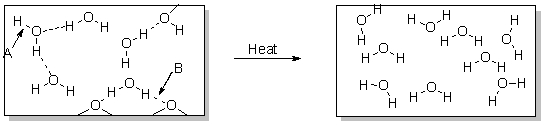
A) Hydrogen bonds are broken.
B) Hydrogen bonds are formed.
C) Polar covalent bonds are broken.
D) Polar covalent bonds are formed.
E) Both hydrogen bonds and polar covalent bonds are broken.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrow A points to 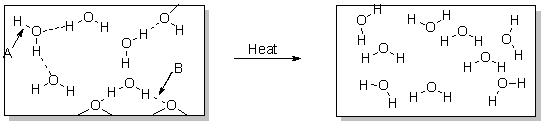
A) a polar covalent bond.
B) a nonpolar bond.
C) a dispersion force.
D) heat.
E) a hydrogen bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements best describes vapor pressure?
A) Vapor pressure is the pressure of a gas at room temperature.
B) Vapor pressure is the pressure of a liquid at room temperature.
C) Vapor pressure is a measure of the strength of a substance's odor.
D) Vapor pressure is a measure of a material's boiling point.
E) Vapor pressure is the pressure of the vapor above a liquid or solid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What change of phase is represented by B on the heating curve? 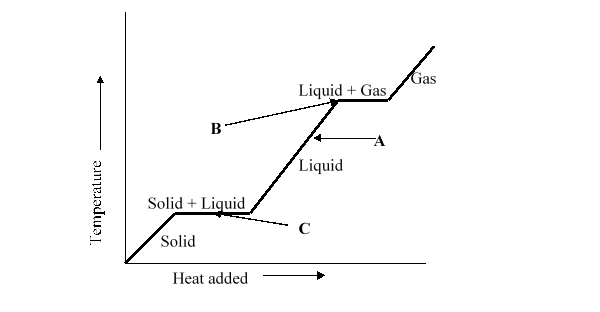
A) boiling
B) freezing
C) sublimating
D) melting
E) It is not possible to determine what will happen to the temperature of the liquid by looking at the chart.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following changes is not correctly labeled as a chemical reaction or a change of state?
A) rust forming on metal, a chemical reaction.
B) steam coming off of a cup of coffee, a change of state.
C) an ice cube melting in a drink, a change of state
D) the combustion of gasoline to give carbon dioxide and water, a chemical reaction
E) bubbles forming in a just opened can of soda, a chemical reaction
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 85
Related Exams