A) Rate = k[CH3CH2CH2Br]
B) Rate = k[CH3CH2CH2Br][KOC(CH3) 3]
C) Rate = k[CH3CH2CH2Br][KOC(CH3) 3]2
D) Rate = k[CH3CH2CH2Br]2[KOC(CH3) 3]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is most likely to react as a nucleophile rather than a base?
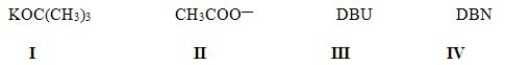
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the major elimination product of the following reaction?
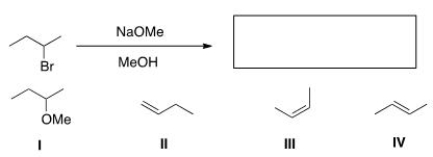
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the elimination product(s) formed by treating the indicated alkyl halide with a base. 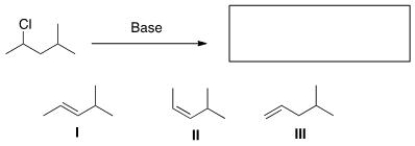
A) Only I
B) Only II
C) Only III
D) I, II, and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the major E2 product formed from the following alkyl halide?
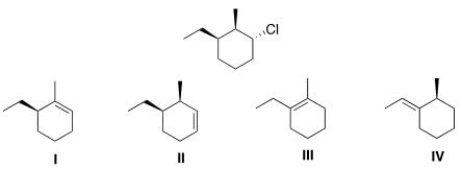
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halides will afford the product below as the major product in an E2 reaction?
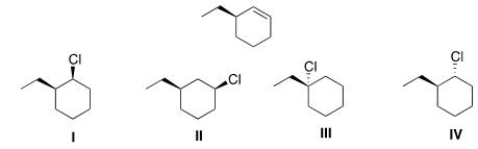
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is (are) the starting material(s) in the reaction below?
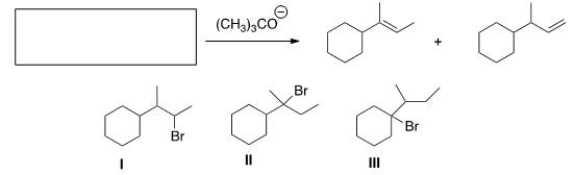
A) Only I
B) Only II
C) Only III
D) II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction?
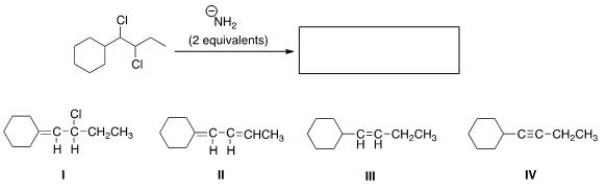
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is (are) the elimination product(s) of the following reaction?
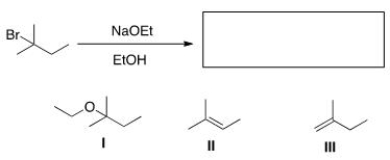
A) Only I
B) Only II
C) Only III
D) II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is (are) the product(s) of the following reaction?
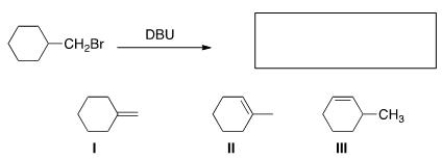
A) Only I
B) Only II
C) Only III
D) I and II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the most reactive substrate in an E1 reaction?

A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the most reactive substrate in an E2 reaction?
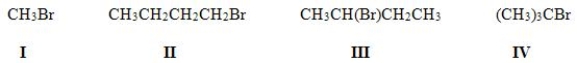
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction?
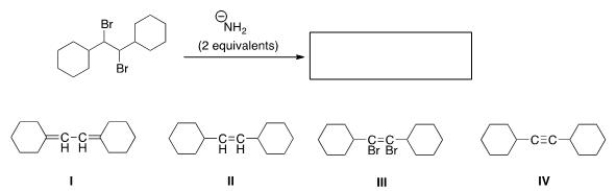
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction?
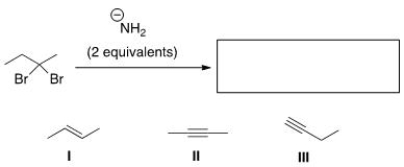
A) Only I
B) Only II
C) Only III
D) II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major elimination product obtained from the following reaction?
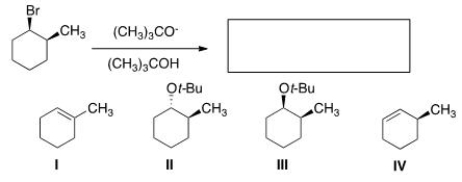
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is (are) the elimination product(s) of the following reaction?
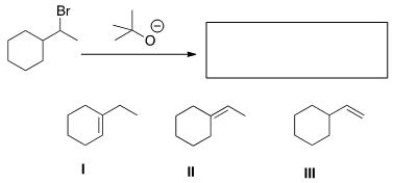
A) Only I
B) Only II
C) Only III
D) II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which alkyl halide(s) would give the following alkene as the only product in an elimination reaction?
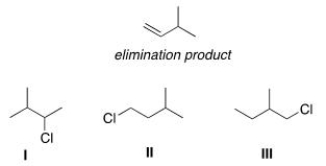
A) Only I
B) Only II
C) Only III
D) I and II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product of the following reaction?
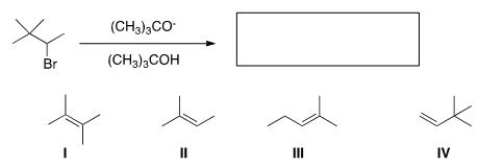
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the mechanism of an E2 reaction is not true?
A) It is fastest with tertiary halides.
B) It exhibits first-order kinetics.
C) A better leaving group should make a faster reaction.
D) All bonds are broken and formed in a single step.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major elimination product obtained from the following reaction?
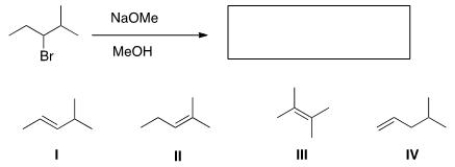
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 43
Related Exams