A) A catalyst accelerates a reaction by changing the amount of reactant and product at equilibrium.
B) A catalyst accelerates a reaction by lowering the energy of activation.
C) A catalyst accelerates a reaction by raising the energy of activation.
D) A catalyst accelerates a reaction by lowering the equilibrium constant.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What kind of reaction does the conversion of A to D represent?
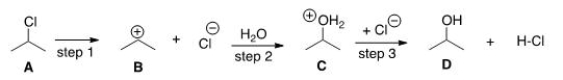
A) Addition reaction
B) Substitution reaction
C) Elimination reaction
D) Oxidation-reduction reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound would you predict to be highest in energy?
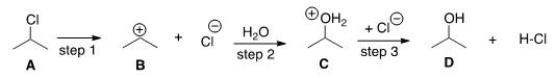
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the equilibrium constant, Keq, is true?
A) When Keq > 1, the equilibrium favors the reactants.
B) When Keq < 1, the equilibrium favors the products.
C) The size of Keq tells about the position of equilibrium.
D) For a reaction to be useful, the equilibrium must favor the reactants.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about addition reactions is true?
A) Two π bonds are formed.
B) Two π bonds are broken.
C) Two σ bonds are formed.
D) One π bond is formed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about bond breaking is not true?
A) Homolysis generates uncharged reactive intermediates with unpaired electrons.
B) Homolysis require energy but heterolysis does not require energy.
C) Heterolysis generates charged intermediates.
D) Heterolysis involves unequal sharing of bonding electrons by atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many transition states and intermediates would the reaction profile have for the reaction shown below?
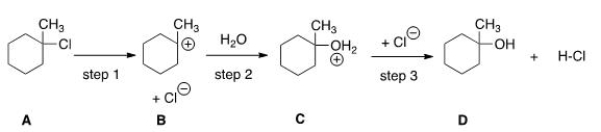
A) Three transition states and three intermediates
B) Two transition states and two intermediates
C) Three transition states and two intermediates
D) Two transition states and three intermediates
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true?
A) Bond breaking is endothermic.
B) The bond dissociation energy for bond breaking is always negative.
C) Bond making is exothermic.
D) The bond dissociation energy for bond formation is always negative.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Using the bond dissociation energies given, calculate DH° for the following reaction. 

A) +108 KJ/mol
B) -130 KJ/mol
C) -22 KJ/mol
D) +22 KJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reaction quantities will have an effect on reaction rate?
A) DG°
B) DH°
C) Keq
D) Ea
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about substitution reactions is true?
A) Substitution reactions involve π bonds.
B) Substitution reactions involve σ bonds.
C) One σ bond breaks and another forms at a different carbon atom.
D) One π bond breaks and another forms at the same carbon atom.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism:
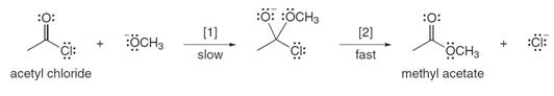 If the concentrations of both -OCH3 and acetyl chloride were increased 2 times, what would happen to the rate of the reaction?
If the concentrations of both -OCH3 and acetyl chloride were increased 2 times, what would happen to the rate of the reaction?
A) Rate would become one-fourth
B) Rate would increase 4 times
C) Rate would increase 16 times
D) Rate would increase 2 times
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about a two-step reaction mechanism is true?
A) The transition states are located at energy minima.
B) Each step is characterized by its own value of DH° and Ea.
C) The rate-determining step has the lower energy transition state.
D) The reactive intermediate is located at an energy maximum.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true?
A) Fast reactions have small rate constants.
B) Slow reactions have large rate constants.
C) A rate equation contains concentration terms for all reactants involved in a one-step mechanism.
D) A rate equation contains concentration terms for all the reactants involved in a multi-step reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true?
A) The product is favored in reaction in which DH° is a positive value.
B) Entropy decreases when an acyclic compound forms a ring.
C) In homolytic bond cleavage, entropy decreases and favors formation of products.
D) The starting material is favored in a reaction in which DH° is a negative value.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which reaction is slowest?
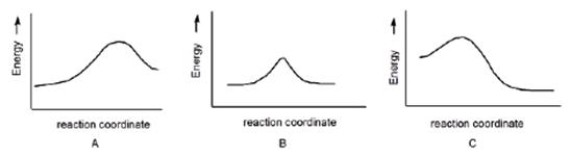
A) A
B) B
C) C
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Calculate Ea for the conversion of C B. 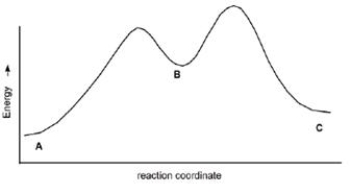
A) +3 kcal
B) +7 kcal
C) +9 kcal
D) None of these
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
A decrease in which of the following results in an increase in the rate of a chemical reaction?
A) Energy of activation
B) Concentration
C) Temperature
D) Kinetic energy
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which reaction has a positive DG°, assuming that entropy changes are negligible compared to enthalpy changes?
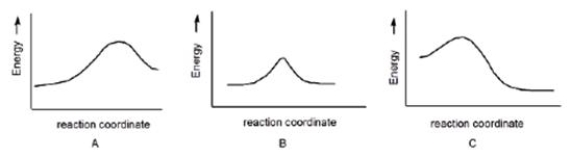
A) A
B) B
C) C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What kind of reaction does the conversion of A to B represent?
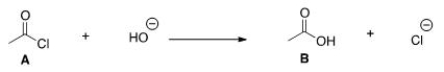
A) Addition reaction.
B) Substitution reaction.
C) Elimination reaction.
D) Acid-base reaction.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 45
Related Exams