A) 3, 3
B) 6, 6
C) 7, 6
D) 5, 4
E) 3, 6
Correct Answer

verified
Correct Answer
verified
True/False
A species that causes a decrease in the oxidation number of another substance is called a reducing agent.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In any electrolytic cell, the cathode is the electrode
A) that attracts anions.
B) at which electrons are collected from electron donors in the solution.
C) at which reduction occurs.
D) at which oxidation occurs.
E) that is made of graphite.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of these statements concerning oxidation are correct except that
A) oxygen is necessary for oxidation to take place.
B) the oxidizing agent receives electrons from another species.
C) oxidation must accompany reduction.
D) the oxidizing agent increases the oxidation number of another element.
E) the oxidation of a metal produces positive ions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The figure shows the electrolysis of molten NaI.What reaction occurs at the anode of this cell? 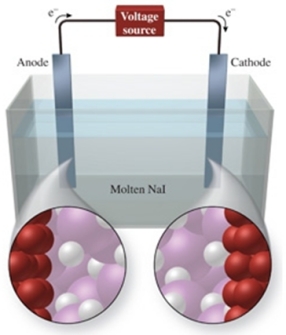
A) NaI(l) Na+(l) + I-(l)
B) NaI(l) Na(l) + I(g)
C) NaI(s) Na+(aq) + I-(aq)
D) 2NaI(s) 2Na(s) + I2(s)
E) 2NaI(l) 2Na(l) + I2(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding oxidation-reduction reactions is correct?
A) Oxidation-reduction reactions involve sharing electrons.
B) Oxidation can occur without reduction.
C) You can tell that a substance is oxidized if it loses electrons.
D) You can tell that a substance is reduced if its oxidation number increases.
E) None of these statements is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider a voltaic cell that corresponds to the following reaction: Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Which of the following statements is correct?
A) Zinc is the oxidizing agent.
B) The Cu2+ solution must be in the half-cell with the zinc electrode.
C) No salt bridge is necessary, since the charge is 2+ on both sides of the equation.
D) The zinc electrode is the cathode.
E) Two electrons will be transferred in this reaction per atom of zinc that reacts.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: H3AsO3(aq) + BiO3-(aq) H3AsO4(aq) + Bi(s) When this equation is balanced in acidic solution, the coefficient for water will be__________, and the number of electrons transferred will be __________.
A) 2, 6
B) 2, 1
C) 1, 10
D) 1, 2
E) 2, 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which compound does phosphorus have an oxidation number of 3+?
A) AlPO4
B) PF5
C) H3PO4
D) H3PO3
E) PH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The change, Br2 + H2O HOBr + HBr, is
A) oxidation only.
B) reduction only.
C) both oxidation and reduction.
D) neither oxidation nor reduction.
E) an acid-base reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction that occurs in an alkaline battery is as follows: Zn(s) + MnO2(s) + H2O(l) ZnO(s) + Mn(OH) 2(s) The substance that is oxidized in this battery is:
A) Zn
B) ZnO
C) MnO2
D) H2O
E) Mn(OH) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A voltaic cell is prepared in which copper metal is oxidized to Cu(II) , and silver ion is reduced to silver metal.Which of the following represents the equation for the reaction that occurs at the anode?
A) Cu(s) - 2e- Cu2+(aq)
B) Cu(s) Cu2+(aq) + 2e-
C) 2Cu(s) 2Cu2+(aq) + e-
D) Ag+(aq) + e- Ag(s)
E) Ag(s) Ag+(aq) + e-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: N2(g) + 3H2(g) 2NH3(g) Which of the following statements is correct?
A) Nitrogen is oxidized.
B) Hydrogen is reduced.
C) Nitrogen is the reducing agent.
D) The reaction is not an oxidation-reduction reaction.
E) Hydrogen is the reducing agent.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: Al(s) + H2O(l) Al(OH) 4-(aq) + H2(g) When this equation is balanced in basic solution, the coefficient for water will be__________, and the number of electrons transferred will be __________.
A) 3, 3
B) 2, 1
C) 6, 8
D) 6, 6
E) 2, 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: Sn2+(aq) + 2Fe3+(aq) Sn4+(aq) + 2Fe2+(aq) Which species is oxidized, and how many electrons are transferred per SN2+ ion that reacts?
A) Sn2+, 2 electrons
B) Fe3+, 2 electrons
C) Sn4+, 4 electrons
D) Fe2+, 4 electrons
E) Fe2+, 2 electrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A lead-acid battery that is used in cars and trucks is powered by the reaction: PbO2(s) + Pb(s) + 2H2SO4(aq) 2PbSO4(s) + H2O(l) The substance that is the reducing agent in this battery is:
A) PbSO4
B) H2O
C) PbO2
D) Pb
E) H2SO4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction, what is the oxidizing agent? 2Cl2(g) + C(s) + 2H2O(l) CO2(g) + 4HCl(aq)
A) Cl2
B) C
C) H2O
D) CO2
E) HCl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of boron in sodium tetraborate, Na2B4O7?
A) +12
B) -3
C) +14
D) +3
E) +4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction: Mg(s) + ZnSO4(aq) MgSO4(aq) + Zn(s) Which of the following statements regarding this reaction is correct?
A) Magnesium is neither oxidized nor reduced.
B) The sulfate ion is reduced.
C) Zinc is the reducing agent.
D) Magnesium is the oxidizing agent.
E) Zinc gains two electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following reaction in a voltaic cell: Zn(s) + Ni2+(aq) Ni(s) + Zn2+(aq) In this cell, Zn(s) is called the
A) anode.
B) oxidizing agent.
C) salt bridge.
D) electrolyte.
E) cathode.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 117
Related Exams