A) 0.020
B) 1.70
C) 7.00
D) 12.30
E) −1.70
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the two Brønsted-Lowry bases in the following equation: CH3NH2(aq) + H2O(l) ⇌ CH3NH3+(aq) + OH−(aq)
A) CH3NH2 and H2O
B) CH3NH2 and OH−
C) H2O and OH−
D) CH3NH2 and CH3NH3+
E) CH3NH3+ and OH−
Correct Answer

verified
Correct Answer
verified
True/False
In polyprotic acids, the first ionizable hydrogen always ionizes to a greater extent than the second or third ionizable hydrogen(s).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The image shows a molecular-level representation of part of a solution after HF is dissolved in water. (Besides water, there are 6 HF molecules, 1 H3O+ ion, and 1 F− ion present in the solution.) Which of the following best describes HF? 
A) strong acid
B) strong base
C) weak acid
D) weak base
E) amphoteric
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following reaction goes in the reverse direction (from products to reactants) , what is the acid? HCN(aq) + H2O(l) ⇌ CN− (aq) + H3O+(aq)
A) HCN
B) H2O
C) CN−
D) H3O+
E) both CN− and H3O+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of a solution that has [H3O+] = 5.2 × 10-7 M.
A) pH = 5.20
B) pH = 6.28
C) pH = 7.00
D) pH = 5.27
E) pH = 7.72
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following equations represents the behavior of H2PO4- as an acid?
A) H2PO4-(aq) + H3O+(aq) ⇌ H3PO4(aq) + H2O(l)
B) H2PO4-(aq) + HF(aq) ⇌ H3PO4(aq) + F-(aq)
C) H2PO4-(aq) + HCN(aq) ⇌ H3PO4(aq) + CN-(aq)
D) H2PO4-(aq) + OH-(aq) ⇌ HPO42-(aq) + H2O(l)
E) H2PO4-(aq) + H2O(l) ⇌ H3PO4(aq) + OH-(aq)
Correct Answer

verified
Correct Answer
verified
True/False
The H3O+ concentration in a 0.050 M solution of HClO4 is 0.050 M.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List the species present in order of increasing concentration in a 0.1 M solution of H2S.
A) H2S < HS- < S2-
B) H2S < S2- < HS-
C) S2- < HS- < H2S
D) HS- < S2- < H2S
E) S2- < H2S < HS-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the molecular-level diagrams to each of the following compounds in aqueous solution: HCl, HF, NH3 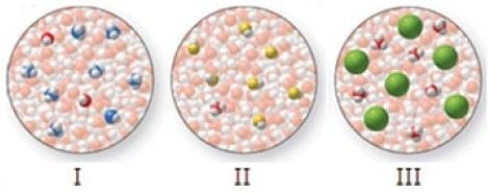
A) I = HCl, II = HF, III = NH3
B) I = HF, II = HCl, III = NH3
C) I = HF, II = NH3, III = HCl
D) I = NH3, II = HF, III = HCl
E) I = NH3, II = HCl, III = HF
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Ka for acetic acid is 1.8 × 10-5. Which of the following statements best describes the pH of a 0.010 M solution of acetic acid?
A) The pH is greater than 0 but less than 2.
B) The pH is exactly 2.
C) The pH is greater than 2 but less than 7.
D) The pH is exactly 7.
E) The pH is greater than 7 but less than 12.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of a solution that has [OH-] = 3.1 × 10-8 M.
A) pH = 3.10
B) pH = 8.00
C) pH = 7.51
D) pH = 3.18
E) pH = 6.49
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following reaction goes in the reverse direction (from products to reactants) , what is the base? HF(aq) + H2O(l) ⇌ F−(aq) + H3O+(aq)
A) HF
B) H2O
C) F−
D) H3O+
E) both F− and H3O+
Correct Answer

verified
Correct Answer
verified
Short Answer
Would you expect the pH of a 0.010 M solution of HF to be less than, greater than, or equal to 2.00? Explain.
Correct Answer

Answered by ExamLex AI
To determine whether the pH of a 0.010 M...View Answer
Show Answer
Correct Answer
Answered by ExamLex AI
View Answer
True/False
The symbol In- can be used to represent the base form of an indicator.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The substance NH3(aq) is
A) a strong acid.
B) a weak acid.
C) a strong base.
D) a weak base.
E) neither an acid nor a base.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the pOH of a coffee sample is 8.85, what is the H3O+ concentration in the coffee?
A) 5.2 M
B) 7.1 × 10-8 M
C) 7.1 × 10-7 M
D) 7.1 × 10-6 M
E) 8.8 M
Correct Answer

verified
Correct Answer
verified
Showing 121 - 137 of 137
Related Exams