A) 10.0 mL
B) 33.3 mL
C) 50.0 mL
D) 83.3 mL
E) 100.0 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
3.00 moles of NaOH (sodium hydroxide) are needed to prepare a solution. What mass of sodium hydroxide is required?
A) 13.3 g
B) 1.20 × 102 g
C) 93 g
D) 10.3 g
E) 1.81 × 1024 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the moles and the mass of solute in 750.0 mL of 3.00 M NaCl.
A) 2.25 moles and 131 g
B) 2.25 moles and 0.0385 g
C) 4.00 moles and 234 g
D) 4.00 moles and 0.0684 g
E) 2.25 × 103 moles and 38.5 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molarity of a solution consisting of 75.0 g of KOH in 2.00 L of solution.
A) 37.5 M
B) 0.938 M
C) 1.34 M
D) 0.668 M
E) 2.10 × 103 M
Correct Answer

verified
Correct Answer
verified
True/False
The substance in the figure with the most atoms per mole is NaCl. 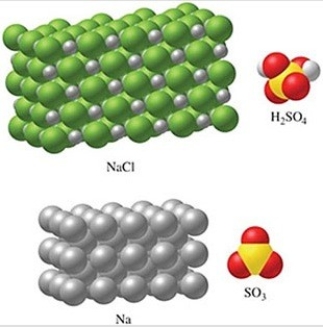
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the number of moles of NaHCO3 (sodium bicarbonate, or baking soda) in a 5.0 g sample of this substance.
A) 0.096 mole
B) 0.060 mole
C) 420 moles
D) 3.6 × 1022 moles
E) 2.8 × 1023 mole
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order from least nitrogen atoms to most nitrogen atoms in a 50.0 g sample: N2, N2O5, NH3, NH4Cl
A) N2 < N2O5 < NH3 < NH4Cl
B) N2O5 < NH4Cl < NH3 < N2
C) N2 < NH3 < NH4Cl < N2O5
D) NH3 < NH4Cl < N2 < N2O5
E) NH4Cl < NH3 < N2 < N2O5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many chloride ions are present in 0.100 mol of MgCl2?
A) 0.200 Cl− ions
B) 6.02 × 1022 Cl− ions
C) 1.20 × 1023 Cl− ions
D) 3.32 × 10−25 Cl− ions
E) 3.01 × 1024 Cl− ions
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much water must be added to 500.0 mL of a 3.00 M HCl solution to obtain a solution that is 2.00 M?
A) 1.00 L
B) 1.50 L
C) 7.50 × 102 mL
D) 2.50 × 102 mL
E) 12.0 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass of 2.4 × 1023 molecules of NO2?
A) 75 g
B) 18 g
C) 1.2 × 102 g
D) 1.1 × 1023 g
E) 12 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding empirical formulas, molecular formulas, and percent composition is correct?
A) A compound with the molecular formula P4O10 would have the empirical formula P4O10.
B) It is not possible to determine the empirical formula of a compound if given only its percent composition.
C) If the molecular formula of a compound is C6H5Cl, its empirical formula is the same.
D) Empirical formulas contain more information than molecular formulas.
E) Formulas for ionic compounds are normally given as molecular formulas.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetic acid is the active ingredient in vinegar. It consists of 40.00% C, 6.714% H, and 53.29% O. What is the empirical formula of acetic acid?
A) C3.33H6.66O3.33
B) C3H6O3
C) CH2O
D) C2H4O2
E) CH3O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Methyl butyrate is a compound that is partially responsible for the flavor of apples. It consists of 58.80% C, 9.87% H, and 31.33% O. What is the empirical formula of methyl butyrate?
A) C6H10O3
B) C5H10O2
C) CH2O
D) C4.9H9.8O1.9
E) C4H9O2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is incorrect?
A) N2H4 has four times as many hydrogen atoms as nitrogen atoms.
B) SF4 has ¼ as many sulfur atoms as fluorine atoms.
C) SO2 has twice as many oxygen atoms as sulfur atoms.
D) Ca(NO3) 2 has six times as many oxygen atoms as calcium ions.
E) H2SO4 has twice as many oxygen atoms as hydrogen atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following sets of formulas is correct for the molecules in the figure? 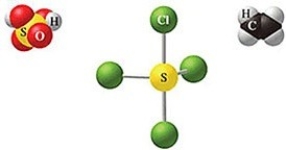
A) H2SO3, SCl4, C2H4
B) H2SO3, SCl3, C2H4
C) H2SO4, SCl3, C2H4
D) H2SO4, SCl4, CH4
E) H2SO4, SCl4, C2H4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many oxygen atoms are in 75.0 g of SO3?
A) 225 atoms
B) 0.937 atoms
C) 5.64 × 1023 atoms
D) 1.69 × 1024 atoms
E) 4.52 × 1025 atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following elements in order from least to most number of moles of atoms in a 10.0 g sample: Br, Fe, Pb, Hg
A) Br < Hg < Fe < Pb
B) Pb < Hg < Br < Fe
C) Fe < Br < Hg < Pb
D) Br < Fe < Hg < Pb
E) Hg < Pb < Br < Fe
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following in order of increasing mass: 2.0 mole of SO2, 1.0 mole of SO3, 0.50 mole of Mo, and 0.25 mole of Rn.
A) SO3 < SO2 < Rn < Mo
B) SO2 < SO3 < Mo < Rn
C) Mo < Rn < SO3 < SO2
D) Mo < Rn < SO2 < SO3
E) Rn < Mo < SO3 < SO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following molecular formulas, determine the empirical formula of each compound: N2O5, PCl3, H2O2, C6H4Cl2.
A) N2O5, PCl3, HO, C6H4Cl2
B) N2O5, PCl3, H2O, C6H4Cl2
C) N2O5, PCl3, H2O2, C3H2Cl2
D) NO2.5, PCl3, HO, C3H2Cl
E) N2O5, PCl3, HO, C3H2Cl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula for novocain, a local anesthetic, is C13H21N2O2. What is the molar mass of this compound?
A) 43.03 g/mol
B) 237.32 g/mol
C) 2035.27 g/mol
D) 38.00 g/mol
E) 4.214 × 10-5 g/mol
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 144
Related Exams