A) C₁₈H₃₆O₁₈
B) C₁₈H₃₂O₁₆
C) C₆H₁₀O₅
D) C₁₈H₁₀O₁₅
E) C₃H₆O₃
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the term used for a protein molecule that assists in the proper folding of other proteins?
A) tertiary protein
B) chaperonin
C) enzyme protein
D) renaturing protein
E) denaturing protein
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a DNA sample were composed of 10% thymine, what would be the percentage of guanine?
A) 10
B) 20
C) 40
D) 80
E) impossible to tell from the information given
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On food packages, to what does the term insoluble fiber refer?
A) cellulose
B) polypeptides
C) starch
D) amylopectin
E) chitin
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following techniques uses the amino acid sequences of polypeptides to predict a protein's three-dimensional structure?
A) X-ray crystallography
B) bioinformatics
C) analysis of amino acid sequence of small fragments
D) NMR spectroscopy
E) high-speed centrifugation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule with the chemical formula C₆H₁₂O₆ is probably a
A) carbohydrate.
B) lipid.
C) monosaccharide
D) carbohydrate and lipid only.
E) carbohydrate and monosaccharide only.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If cells are grown in a medium containing radioactive ³²P-labeled phosphate, which of these molecules will be labeled?
A) phospholipids
B) nucleic acids
C) proteins
D) amylose
E) both phospholipids and nucleic acids
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bonding of two amino acid molecules to form a larger molecule requires which of the following?
A) removal of a water molecule
B) addition of a water molecule
C) formation of a glycosidic bond
D) formation of a hydrogen bond
E) both removal of a water molecule and formation of a hydrogen bond
Correct Answer

verified
Correct Answer
verified
Multiple Choice
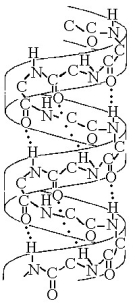 Figure 5.7
-The structure depicted in Figure 5.7 shows the
Figure 5.7
-The structure depicted in Figure 5.7 shows the
A) 1-4 linkage of the α glucose monomers of starch.
B) 1-4 linkage of the β glucose monomers of cellulose.
C) double-helical structure of a DNA molecule.
D) α helix secondary structure of a polypeptide.
E) β pleated sheet secondary structure of a polypeptide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following questions are based on the 15 molecules illustrated in Figure 5.8. Each molecule may be used once, more than once, or not at all.
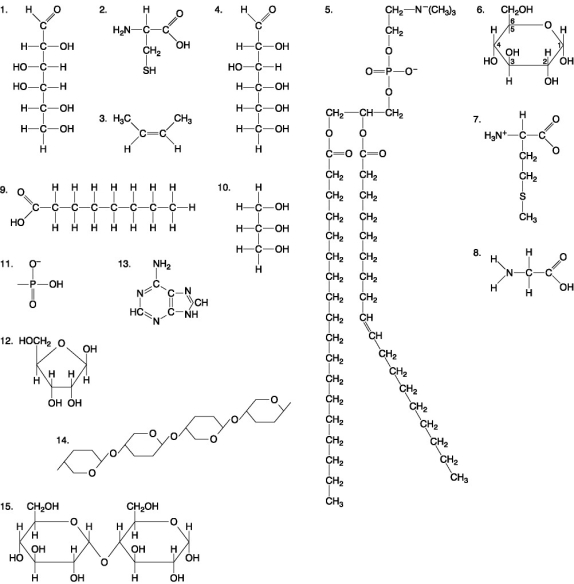 Figure 5.8
-Which of the following molecules could be joined together by a peptide bond as a result of a dehydration reaction?
Figure 5.8
-Which of the following molecules could be joined together by a peptide bond as a result of a dehydration reaction?
A) 2 and 3
B) 3 and 7
C) 7 and 8
D) 8 and 9
E) 12 and 13
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A double-stranded DNA molecule contains a total of 120 purines and 120 pyrimidines. This DNA molecule could be composed of
A) 120 adenine and 120 uracil molecules.
B) 120 thymine and 120 adenine molecules.
C) 120 cytosine and 120 thymine molecules.
D) 120 adenine and 120 cytosine molecules.
E) 120 guanine and 120 thymine molecules.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
There are 20 different amino acids. What makes one amino acid different from another?
A) different side chains (R groups) attached to a carboxyl carbon
B) different side chains (R groups) attached to the amino groups
C) different side chains (R groups) attached to an α carbon
D) different structural and optical isomers
E) different asymmetric carbons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an example of hydrolysis?
A) the reaction of two monosaccharides, forming a disaccharide with the release of water
B) the synthesis of two amino acids, forming a peptide with the release of water
C) the reaction of a fat, forming glycerol and fatty acids with the release of water
D) the reaction of a fat, forming glycerol and fatty acids with the consumption of water
E) the synthesis of a nucleotide from a phosphate, a pentose sugar, and a nitrogenous base with the production of a molecule of water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
 Figure 5.1
-If two molecules of the general type shown in Figure 5.1 were linked together, carbon-1 of one molecule to carbon-4 of the other, the single molecule that would result would be
Figure 5.1
-If two molecules of the general type shown in Figure 5.1 were linked together, carbon-1 of one molecule to carbon-4 of the other, the single molecule that would result would be
A) maltose.
B) fructose.
C) glucose.
D) galactose.
E) sucrose.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
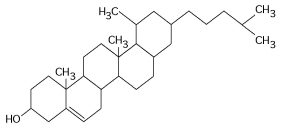 Figure 5.4
-What is the structure shown in Figure 5.4?
Figure 5.4
-What is the structure shown in Figure 5.4?
A) pentose molecule
B) fatty acid molecule
C) steroid molecule
D) oligosaccharide molecule
E) phospholipid molecule
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Upon chemical analysis, a particular polypeptide was found to contain 100 amino acids. How many peptide bonds are present in this protein?
A) 101
B) 100
C) 99
D) 98
E) 97
Correct Answer

verified
Correct Answer
verified
Multiple Choice
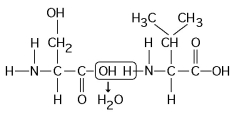 Figure 5.5
-Which of the following statements is/are true regarding the chemical reaction illustrated in Figure 5.5?
Figure 5.5
-Which of the following statements is/are true regarding the chemical reaction illustrated in Figure 5.5?
A) It is a hydrolysis reaction.
B) It results in a peptide bond.
C) It joins two fatty acids together.
D) It is a hydrolysis reaction and it results in a peptide bond.
E) It is a hydrolysis reaction, it results in a peptide bond, and it joins two fatty acids together.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The tertiary structure of a protein is the
A) bonding together of several polypeptide chains by weak bonds.
B) order in which amino acids are joined in a polypeptide chain.
C) unique three-dimensional shape of the fully folded polypeptide.
D) organization of a polypeptide chain into an α helix or β pleated sheet.
E) overall protein structure resulting from the aggregation of two or more polypeptide subunits.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following questions are based on the 15 molecules illustrated in Figure 5.8. Each molecule may be used once, more than once, or not at all.
 Figure 5.8
-A fat (or triacylglycerol) would be formed as a result of a dehydration reaction between
Figure 5.8
-A fat (or triacylglycerol) would be formed as a result of a dehydration reaction between
A) one molecule of 9 and three molecules of 10.
B) three molecules of 9 and one molecule of 10.
C) one molecule of 5 and three molecules of 9.
D) three molecules of 5 and one molecule of 9.
E) one molecule of 5 and three molecules of 10.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the chemical reaction mechanism by which cells make polymers from monomers?
A) phosphodiester linkages
B) hydrolysis
C) dehydration reactions
D) ionic bonding of monomers
E) the formation of disulfide bridges between monomers
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 109
Related Exams