Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following contains ionic bonding?
A) CO
B) SrF2
C) Al
D) OCl2
E) HCl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following species does the central atom violate the octet rule?
A) CH4
B) SF4
C) PCl4+
D) CCl3+
E) NH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these compounds is most likely to be ionic?
A) GaAs
B) SrBr2
C) NO2
D) CBr4
E) H2O
Correct Answer

verified
Correct Answer
verified
Short Answer
In the Lewis dot symbol, the element symbol represents the ________ and the dots represent the ________.
Correct Answer

verified
core elect...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The Lewis dot symbol consists of the symbol for the element surrounded by dot(s) . What does the dot or dots represent?
A) Electron configuration
B) Valence electrons
C) Atomic number
D) Atomic mass
E) Core electrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an element is bonded to 4 other atoms and has a formal charge of +1, what group must the element be in?
A) 3A
B) 4A
C) 5A
D) 6A
E) 7A
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis structure for a chlorate ion, ClO3-, should show ________ single bond(s) , ________ double bond(s) , and ________ lone pair(s) .
A) 1, 2, 7
B) 3, 0, 9
C) 2, 1, 9
D) 3, 0, 10
E) 1, 2, 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct Lewis structure for nitrogen trifluoride, NF3.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an ionic compound?
A) H2S
B) NH3
C) I2
D) KI
E) CCl4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis dot symbol for the a lead atom is
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of resonance structures for the sulfur dioxide molecule that satisfy the octet rule is
A) 1.
B) 2.
C) 3.
D) 4.
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the resonance structure of sulfur dioxide, the sulfur atom has one single bond and one double bond. What is the formal charge of this sulfur atom?
A) 0
B) +1
C) -1
D) +2
E) -2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the element whose Lewis symbol is correct.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the Lewis structure of the iodate ion, IO3-, that satisfies the octet rule, the formal charge on the central iodine atom is
A) +2.
B) +1.
C) 0.
D) -1.
E) -2.
Correct Answer

verified
Correct Answer
verified
Short Answer
The number of dots in the Lewis dot symbol for a main group element is the same as the ________ ________.
Correct Answer

verified
group numb...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the formal charge on phosphorus in a Lewis structure for the phosphate ion that satisfies the octet rule?
A) -2
B) -1
C) 0
D) +1
E) +2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following Lewis structures is definitely incorrect?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following Lewis structure for ClO3F, chlorine has a formal charge of ________ and an oxidation number of ________. 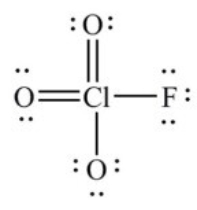
A) 7, 7
B) 7, -1
C) 1, 1
D) 1, -1
E) 1, 7
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many total resonance structures can be drawn for the sulfate ion, SO42-?
A) 2
B) 3
C) 4
D) 5
E) 6
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 102
Related Exams