A) 224 moles
B) 2.24 moles
C) 0.0446 moles
D) 0.446 moles
E) 22.4 moles
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The temperature of a 350 mL sample of gas increases from 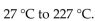 What is the final volume of the sample of gas, if the pressure and amount of gas in the container is kept constant?
What is the final volume of the sample of gas, if the pressure and amount of gas in the container is kept constant?
A) 41.6 mL
B) 2940 mL
C) 583 mL
D) 110. mL
E) 210 .mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A balloon is filled with helium gas. For the question(s) that follow, select the letter of the balloon diagram that corresponds to the given
change in conditions. 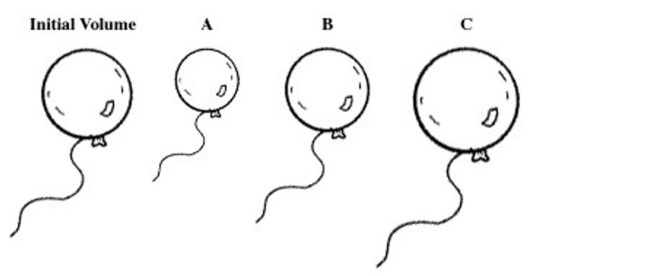 -The balloon is put into a chamber whose pressure is less than the atmospheric pressure and at atmospheric temperature.
-The balloon is put into a chamber whose pressure is less than the atmospheric pressure and at atmospheric temperature.
A) A
B) B
C) C
D) A and B
E) B and C
Correct Answer

verified
Correct Answer
verified
Essay
The pressure unit 1 mmHg is the same pressure unit as the pressure unit ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pressure, in mmHg, of a 4.00 g sample of 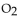 gas, which has a temperature of
gas, which has a temperature of 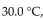 and a volume of 3.00 L?
and a volume of 3.00 L?
A) 78.0 mmHg
B) 0.788 mmHg
C) 7880 mmHg
D) 1.04 mmHg
E) 788 mmHg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass of neon that exerts a pressure of 720. mmHg, with a temperature of -15.0 °C , when the volume of the container is 760. mL?
A) 0.0340 g
B) 0.615 g
C) 0.686 g
D) 517 g
E) 25.8 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass of a sample of O2 gas, which has a pressure of 740. mmHg, at a temperature of 25 °C, in a volume of 250. mL?
A) 320. g
B) 292 g
C) 0.318 g
D) 201 g
E) 3.82 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At STP, what is the volume of 4.50 moles of nitrogen gas?
A) 3420 L
B) 1230 L
C) 60.7 L
D) 101 L
E) 167 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The force of the collisions of gas particles against the walls of a container is called
A) quantity of gas.
B) temperature.
C) volume.
D) density.
E) pressure.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas sample in a closed, expandable container of initial volume 5.00 L was allowed to warm from 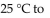
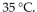 at constant amount of gas and pressure. What was its new volume?
at constant amount of gas and pressure. What was its new volume?
A) 4380 L
B) 7.00 L
C) 3.57 L
D) 4.84 L
E) 5.17 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a gas mixture, the partial pressures are:  76.0 torr, He 230 mmHg , and
76.0 torr, He 230 mmHg , and 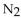 0.640 atm. The total pressure in mmHg is
0.640 atm. The total pressure in mmHg is
A) 792 mmHg
B) 306 mmHg
C) 760 mmHg
D) 562 mmHg
E) 486 mmHg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete the following statement: According to Charles's Law, the volume of a gas ________ when the ________ decreases.
A) increases; temperature
B) increases; quantity of gas
C) decreases; temperature
D) decreases; pressure
E) increases; pressure
Correct Answer

verified
Correct Answer
verified
Essay
Boyle's law is usually written as ________.
Correct Answer

verified
Correct Answer
verified
Essay
The use of high-pressure chambers to control disease processes is known as ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to Gay-Lussac's Law, the pressure of a gas increases due to an increase in temperature because
A) the molecules strike the walls of the container more often and with more force.
B) there is an increase in the number of gas particles.
C) there is a decrease in the volume of the container.
D) the molecules strike the walls of the container less often and with less force.
E) the molecules get bigger.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At STP, 11 g of 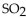 has a volume of
has a volume of
A) 3.8 L .
B) 130 L.
C) 22 L.
D) 0.0076 L.
E) 250 L.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
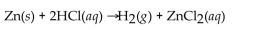 When 25.0 g of Zn reacts, how many L of H2 gas are formed at STP?
When 25.0 g of Zn reacts, how many L of H2 gas are formed at STP?
A) 0.382 L
B) 22.4 L
C) 0.0171 L
D) 8.56 L
E) 4.28 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of a gas with an initial pressure of 380 mmHg atm increases from 5.0 L to 8.0 L. What is the final pressure of the gas, in atm, assuming no change in amount of gas or temperature?
A) 2.4 atm
B) 0.31 atm
C) 240 atm
D) 8.0 atm
E) 0.80 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
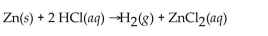 When 25.0 g of Zn reacts, how many L of
When 25.0 g of Zn reacts, how many L of 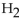 gas are formed at
gas are formed at  and a pressure of 854 mmHg?
and a pressure of 854 mmHg?
A) 22.4 L
B) 8.32 L
C) 0.120 L
D) 0.382 L
E) 8.56 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At STP, what is the mass of 11.2 liters of O2 gas?
A) 32.0 g
B) 128 g
C) 16.0 g
D) 64.0 g
E) 8.00 g
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 84
Related Exams