A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The common name for 2-butanone, a readily available solvent, is
A) ![]()
B) butyl ketone.
C) methyl acetone.
D) methyl ethyl ketone.
E) butyl ether.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The addition of hydrogen to an organic compound or the loss of oxygen is called
A) halogenation.
B) oxidation.
C) dehydration.
D) reduction.
E) hydration.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In all aldehydes except formaldehyde, how many hydrogen atoms are bonded to the carbonyl group?
A) one
B) two
C) three
D) four
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is an aldehyde?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) CH3-CH2-O-CH2-CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The product of adding two molecules of an alcohol to one molecule of an aldehyde in the presence of acid is a(n)
A) hemiacetal.
B) ether.
C) hemiether.
D) acetal.
E) hydroxyl group.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the common name of this compound? 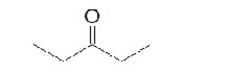
A) acetone
B) 3-pentanone
C) ethyl methyl ketone
D) dimethyl ketone
E) diethyl ketone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would NOT be water soluble?
A) acetone
B) propanal
C) 2-butanone
D) formaldehyde
E) 3-heptanone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Formaldehyde is used industrially to make
A) carpeting.
B) polymers.
C) insulating materials.
D) All of the above.
E) None of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of this compound? 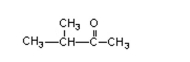
A) 3-methylbutanone
B) 2-methyl-3-butanone
C) 2-methylbutanone
D) pentanone
E) 3-methyl-2-butanone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the bond angles in a typical carbonyl group?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of this compound?
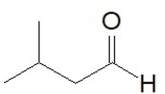
A) 3-methylbutanal
B) 2-methylbutanone
C) 3-methylbutanone
D) pentanone
E) 2-methyl-1-butanal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the compounds would give a positive Tollens' test?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of an alcohol are needed to react with 1 mole of an aldehyde to form a hemiacetal?
A) 3.5 moles
B) 3 moles
C) 1.5 moles
D) 2 moles
E) 1 moles
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The oxygen atom in a carbonyl group is ________ the carbon atom.
A) more electropositive than
B) more soluble than
C) more electronegative than
D) identical in electronegativity to
E) less electronegative than
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hydrogenation of 2-methylpropanal yields
A) 2-methyl-2-propanol.
B) 2-methyl-3-propanol.
C) 1-butanol.
D) 2-methylpropanoic acid.
E) 2-methyl-1-propanol.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An acetal is formed from two molecules of an alcohol and a(n)
A) ester.
B) ether.
C) carboxylic acid.
D) aldehyde.
E) alkyl ether.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How do sugars form cyclic hemiacetals?
A) Functional groups within molecule of sugar react with each other.
B) Two molecules of a sugar react with one another.
C) A molecule of sugar reacts with an added aldehyde.
D) A sugar molecule decomposes.
E) A molecule of sugar reacts with an added alcohol.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetone can be produced by the body when a person is
A) recovering from surgery.
B) sleeping.
C) exercising.
D) dieting with high protein diets.
E) ill with a flu.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reduction of 3-pentanone with hydrogen in the presence of a nickel catalyst will yield
A) pentanaldehyde.
B) 3-pentanol.
C) diethyl alcohol.
D) pentane.
E) 2-pentene.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 44
Related Exams