A) Organic compounds are produced by living things.
B) Unlike inorganic compounds, organic compounds can be cyclic.
C) Carbon atoms can form stable covalent bonds with other carbon atoms.
D) Organic compounds can be larger than inorganic compounds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The naming of organic compounds is specific. Which is an incorrect statement?
A) Many compounds have been known long enough to have common names.
B) The naming system of organic compounds is set by IUPAC rules.
C) The naming system requires that the longest carbon chain must always be found.
D) Organic compounds must have both an IUPAC and a common name.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Due to the method used for treatment, which of the following might be found in drinking water?
A) methyl sulfide
B) chloromethane
C) formaldehyde
D) bromobenzene
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds can exhibit structural isomerism?
A) propane
B) methane
C) butane
D) ethane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the correct statement: The alkanes represent one family of organic compounds
A) in which the halogens are a major component.
B) in which there is at least one double bond between carbons.
C) that is composed of carbon and hydrogen.
D) in which hydrogens can be bonded to other hydrogens.
Correct Answer

verified
Correct Answer
verified
Short Answer
_____ is a property that is characterized by compounds that have identical molecular formulas but different arrangements of atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a structural isomer of the compound shown below? 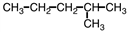
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the IUPAC name for the following chemical. 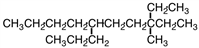
A) 3,6-diethyl-3-methylnonane
B) 2,2,5,6-tetraethylhexane
C) 2,2,5-triethyloctane
D) 3-ethyl-3-methyl-6-propyldecane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Alkanes are ____ in water and ____ than water.
A) insoluble, less dense
B) soluble, less dense
C) insoluble, more dense
D) soluble, more dense
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following terms applies to the alkanes?
A) hydrophobic
B) hydrophilic
C) soluble
D) bases
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What the correct IUPAC name for the compound shown? 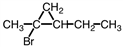
A) 1-bromo-3-ethyl-1-methylcyclopropane
B) 1-bromo-2-ethyl-1-methylcyclopropane
C) 1-bromo-1-ethyl-2-methylcyclopropane
D) 2-bromo-1-ethyl-2-methylcyclopropane
Correct Answer

verified
Correct Answer
verified
True/False
Functional groups are to organic chemistry as monoatomic ions are to inorganic chemistry.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which applies to the manner in which carbon bonds in alkanes?
A) The bonds are formed with carbon using an sp 3 configuration.
B) The bonds between carbon and other atoms are ionic.
C) Carbon to carbon bonds are expected to be double bonds.
D) The bonds formed by carbon all use p orbitals.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Organic chemistry is the branch of chemistry involved with carbon based compounds. Carbon is a member of which group of elements on the periodic table?
A) transition
B) inner transition
C) representative
D) noble
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following is the structural formula of 1-bromo-2,2-dimethylpropane?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
A
Correct Answer
verified
True/False
One of the characteristics of hydrocarbons is that they range from slightly soluble in water to very soluble in water.
Correct Answer

verified
Correct Answer
verified
True/False
Carbon atom tends to form tetrahedral structures and carbon is the basis of organic compounds, so, all organic compounds tend to be tetrahedral in shape.
Correct Answer

verified
Correct Answer
verified
True/False
Since all alkanes are saturated hydrocarbons, all of the carbon atoms are aligned in one continuous chain.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds would be classified as inorganic?
A) CH3CH2OH
B) CH2ClCH2Cl
C) NaC2H3O2
D) C5H12
Correct Answer

verified
Correct Answer
verified
True/False
The major use for hydrocarbons is combustion for energy.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 92
Related Exams