Correct Answer

verified
electrons
...View Answer
Show Answer
Correct Answer
verified
...
View Answer
Short Answer
oxygen
The following is the Lewis structure for the compound commonly known as acetylene 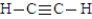 Fill in the blanks with the appropriate integer including zero if necessary (0, 1, 2, 3, etc.).
-There are____________________single bonds in acetlyene.
Fill in the blanks with the appropriate integer including zero if necessary (0, 1, 2, 3, etc.).
-There are____________________single bonds in acetlyene.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An element with the following Lewis structure would most likely form how many covalent bonds? 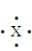
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the molecule shown below. ClCFBrI Which element in this molecule is the central atom?
A) Cl
B) C
C) F
D) Br
E) I
Correct Answer

verified
Correct Answer
verified
Short Answer
Write the name that corresponds to the formula given below. NF3
Correct Answer

verified
nitrogen t...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The anion in an ionic compound whose name ends in "-ide" is
A) always a monoatomic ion.
B) always a polyatomic ion.
C) either a monoatomic or polyatomic ion.
D) positively charged.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a correct electron dot formula for ICl?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
F) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct formula of an ionic compound containing Fe2+ and ClO 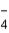 is
is
A) FeClO4
B) Fe2(ClO4) 3
C) Fe(ClO4) 2
D) Fe3(ClO4) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In order to satisfy the octet rule, an atom with the following electron arrangement can shell 1: 2 electrons shell 2: 8 electrons shell 3: 5 electrons
A) gain three electrons.
B) gain two electrons.
C) gain one electron.
D) lose three electrons.
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structure for the following using lines to represent bonding electron pairs. CS2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for K2SO4 is
A) potassium sulfate.
B) potassium sulfide.
C) potassium sulfite.
D) potassium tetrasulfoxygen.
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structure for the following using lines to represent bonding electron pairs. H2O2
Correct Answer

verified
Correct Answer
verified
True/False
The element Co (cobalt) satisfies the octet rule.
Correct Answer

verified
Correct Answer
verified
True/False
In the following compound, phosphorus (P) would be at the center of the molecule. PBrClF
Correct Answer

verified
Correct Answer
verified
Short Answer
Which atom will be positively charged (if any) in the following bond? Enter the symbol for the element or none as appropriate in the blank. P-P ________
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass of 1.00 mol of sulfur trioxide?
A) 48.06 g
B) 80.06 g
C) 32.06 g
D) 112.18 g
Correct Answer

verified
Correct Answer
verified
True/False
18.01 g of water (H2O) and 36.46 g of HCl contain the same number of molecules.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the species formed when a bromine atom gains an electron?
A) bromate
B) bromide ion
C) bromine
D) bromine ion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula for the compound named as tetraphosphorus decoxide? Assume "deca" represents 10.
A) P4O10
B) P2O5
C) P10O4
D) PO4
Correct Answer

verified
Correct Answer
verified
Short Answer
Completion Phosgene is a compound used as a building block of some pharmaceuticals. It contains two chlorine atoms, one oxygen atom, and one carbon atom. Complete the following statement using the appropriate chemical symbol for the elements. -In the Lewis structure for phosgene, there is a double bond between_____________________and ____________________.
Correct Answer

verified
C, O
O, C
...View Answer
Show Answer
Correct Answer
verified
O, C
...
View Answer
Showing 61 - 80 of 83
Related Exams