A) Bloodstream
B) Lipid membrane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the pKa for ammonium ion is 9.26, what is the pH of 1 L of Solution which contains 5.35 g of NH4Cl, and contains the ammonium ion at a concentration of 0.2 mol L-1? (Molar mass of NH4Cl = 54.5 g mol-1.)
A) 7.00
B) 7.65
C) 8.96
D) 9.56
E) 9.95
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following depicts a neutralization reaction?
A) CH3CH2COOH ⇌ CH3CH2COO- + H+
B) CH3CH2COOH + NaOH CH3CH2COONa + H2O
C) CH3CH2COOH + CH3OH CH3CH2COOCH3 + H2O
D) CH3CH2COOH + H2O ⇌ CH3CH2COO- + H3O+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about buffer solutions are true? Select any that apply.
A) A buffer solution must contain a strong acid.
B) A buffer solution undergoes a neutralization reaction to consume excess OH- ions.
C) The conjugate acid present in the buffer solution consumes excess H+ ions.
D) The components of the buffer solution must be present at high concentrations.
E) A buffer solution can only resist changes in pH when small quantities of an acid or base are added to it.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following amino acids with the most appropriate description of the extent of ionization of their side chains when the pH of their environment has a value of 10.0 -Lysine (pKa 10.5)
A) Roughly 50% ionized
B) Less than 50% ionized
C) More than 50% ionized
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Look at the following equilibrium reaction:
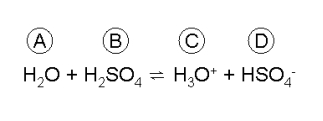 Match each chemical species with the term that best describes its role in this reaction scheme.
-Base
Match each chemical species with the term that best describes its role in this reaction scheme.
-Base
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following acids would you use to make an effective buffer for a reaction at pH 8?
A) Ethanoic acid; Ka = 1.7 10-5
B) Diphosphoric acid; Ka = 1.4 10-7
C) Tris-hydroxmethylaminoethane; Ka = 8.3 10-9
D) Ammonium chloride; Ka = 5.6 10-10
E) Sodium bicarbonate; Ka = 5.3 10-11
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is the correct expression for the ion product of water?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) log pH log pOH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution of HCl at concentration of 4 10-4 mol L-1 has a pH of which of the following?
A) 2.67
B) 3.21
C) 3.40
D) 4.31
E) 4.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of human blood is 7.4. Therefore the hydrogen ion concentration in human blood is which of the following?
A) 7.4 M
B) 2.5 107 M
C) 1.6 103 M
D) 4.0 10-8 M
E) 7.4 �� 10-7 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the weakest acid?
A) Boric acid, Ka = 7.3 10-10
B) Benzoic acid, Ka = 6.3 10-5
C) Sulfurous acid, Ka = 1.2 10-2
D) Ethanoic acid, Ka = 1.8 10-5
E) Formic acid, Ka = 1.8 10-4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Look at the following equilibrium reaction:
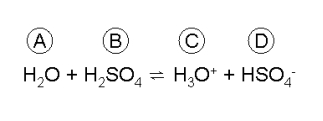 Match each chemical species with the term that best describes its role in this reaction scheme.
-Acid
Match each chemical species with the term that best describes its role in this reaction scheme.
-Acid
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the concentration of [OH-] in an aqueous solution is 0.3 M, what is the pH of that solution?
A) 8.96
B) 10.87
C) 1.45
D) 3.67
E) 13.48
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions depict the behaviour of an acid? Select any that apply.
A) NH3 + H+ ⇌ NH4+
B) CH3OOH- + ⇌CH3COOH
C) CH3COOH ⇌ CH3COO- + H+
D) H2O ⇌ H+ + OH-
E) H2O + H+ ⇌ H3O+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 0.1 M solution of ethanoic acid is 1.34% ionized. What is the value of Ka for ethanoic acid?
A) Ka = 5.3 10-11
B) Ka = 1.82 10-5
C) Ka = 1.34 10-6
D) Ka = 2.67 10-2
E) Ka = 3.01 10-4
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Match the type of compound with the biological environment into which it is most likely to partition. -Strong acid
A) Bloodstream
B) Lipid membrane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following amino acids with the most appropriate description of the extent of ionization of their side chains when the pH of their environment has a value of 10.0 -Arginine (pKa 12.5)
A) Roughly 50% ionized
B) Less than 50% ionized
C) More than 50% ionized
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of a 0.005 M solution of hydrobromic acid is which of the following?
A) 1.3
B) 2.1
C) 2.3
D) 2.0
E) 5.0
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Match the following amino acids with the most appropriate description of the extent of ionization of their side chains when the pH of their environment has a value of 10.0 -Glutamic acid (pKa 4.1)
A) Roughly 50% ionized
B) Less than 50% ionized
C) More than 50% ionized
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Look at the following equilibrium reaction:
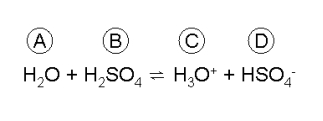 Match each chemical species with the term that best describes its role in this reaction scheme.
-Conjugate acid
Match each chemical species with the term that best describes its role in this reaction scheme.
-Conjugate acid
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 21
Related Exams