A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the classification of the reaction shown? 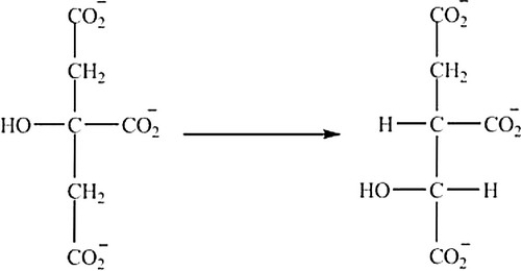
A) Hydrolysis
B) Decarboxylation
C) Isomerization
D) Reduction
E) Oxidation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which intermediate in the citric acid cycle contains two chirality centers?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
FAD is the abbreviation for
A) fluorine adenine dinucleotide.
B) flavin adenine dinucleotide.
C) fluorine amino dinucleotide.
D) flavin amino dinucleotide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement best describes how the interconversion of ATP and ADP is responsible for storing and providing energy for cellular reactions? 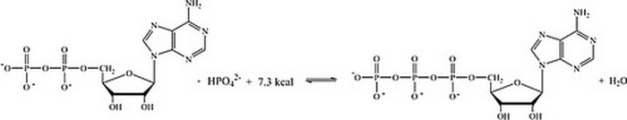
A) The energy required to phosphorylate ADP is stored in ATP, and released when ATP undergoes hydrolysis.
B) The energy required to hydrolyze ATP comes from the energy producing reactions in the cell; the energy lost is then stored in ATP.
C) The energy stored in ADP is released when ATP is synthesized.
D) The energy released in the phosphorylation of ADP can be coupled with unfavorable reactions within the cell.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the classification of the reaction shown? 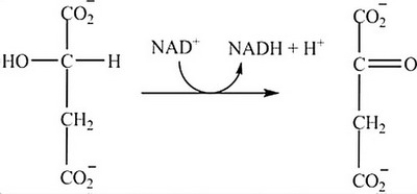
A) Isomerization
B) Hydrolysis
C) Redox
D) Decarboxylation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Step 7 of the citric acid cycle is shown. Which statement best describes what occurs in this step? 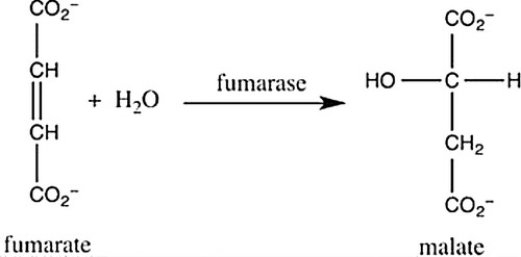
A) Fumarate undergoes reduction with the aid of the cofactor fumarase.
B) Fumarate undergoes hydrogenation with hydrogens and electrons provided by the enzyme fumarase.
C) Fumarate undergoes hydrolysis with the aid of the enzyme fumarase.
D) Fumarate undergoes hydration with the aid of the enzyme fumarase.
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Step 6 of the citric acid cycle is shown. Which statement best describes the role of FAD in this reaction? 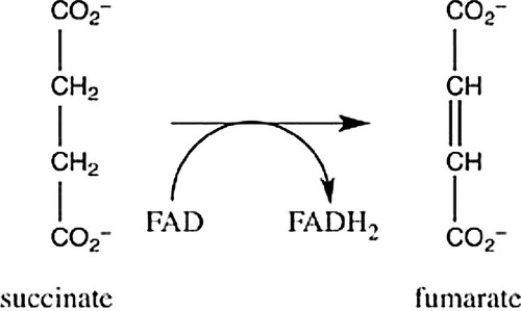
A) FAD causes the oxidation of succinate to form fumarate.
B) FAD causes the isomerication of succinate to form fumarate.
C) FAD causes the reduction of succinate to form fumarate.
D) FAD causes succinate to undergo hydration to form fumarate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the third stage of catabolism?
A) Glycolysis
B) Digestion
C) Formation of acetyl CoA
D) Fatty acid oxidation
E) Citric acid cycle
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the primary function of the citric acid cycle in metabolism?
A) To break down food molecules into smaller components so they can be absorbed by the blood
B) To synthesize ATP from the energy produced in the hydrolysis of citric acid
C) To convert acetyl groups to CO2 molecules and provide reduced coenzymes for the electron transport chain
D) To provide the enzymes necessary to aid in the hydrolysis of carbohydrates, proteins, and lipids
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
The conversion of Fe3+ to Fe2+ in the electron transport chain is an example of Fe3+ acting as a (an) ________ agent.
A) oxidizing
B) oxidizing and reducing
C) catalytic
D) reducing
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the second stage of catabolism?
A) Glycolysis
B) Fatty acid oxidation
C) Citric acid cycle
D) Digestion
E) Formation of acetyl CoA
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule contains the largest amount of stored energy?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are donated by each NADH in the electron transport chain?
A) 0
B) 3
C) 2
D) 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which intermediate in the citric acid cycle is a secondary alcohol?
A) ![]()
B) Two of the intermediates are secondary alcohols.
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxaloacetate is the starting material in the first step of the citric acid cycle and the product of the last step. The last step of the citric acid cycle, the oxidation of malate to oxaloacetate, is shown. What is the structure of oxaloacetate? 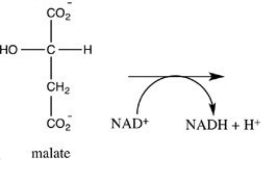
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The second step of the citric acid cycle, the isomerization of citrate to isocitrate, is shown. What is the structure of isocitrate? 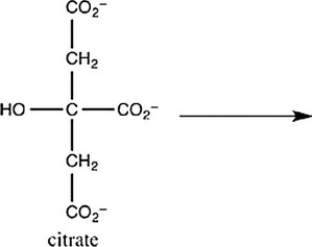
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which is the abbreviated structure of acetyl CoA?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 1 - 18 of 18
Related Exams