Correct Answer

verified
Correct Answer
verified
True/False
The energy of the bond holding two covalently bound atoms together is independent of the distance between the two atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the H°f data given, which of the following compounds is the most unstable?
A) SrO(s) , -592.0 kJ/mol
B) CsBr(s) , -396 kJ/mol
C) CaF2(s) , -1215 kJ/mol
D) NaI(s) , -288 kJ/mol
E) KF(s) , -568.6 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure of CH3NO2.Based on its structure, the nitromethane molecule should have ________ resonance hybrids and ________ atoms with formal charges on them. Hint: Consider possible double and triple bonds in the structure and be sure to minimize formal charge.
A) no, 2
B) no, 3
C) 2, 2
D) 2, 3
E) 3, 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for HClO3. If the valence shells are filled to the usual limit (a maximum of 8) , what is the sum of the absolute values of all the formal charges in the molecule? Hint: Minimize formal charge to ensure you have the most stable or "best"Lewis structure.
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the "best"Lewis structure from formal charge considerations, how many resonance structures, if any, can be drawn for the PO43- ion?
A) 1 (no resonance structures)
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lithium fluoride has a lattice energy of -1033 kJ/mol. In the ionic solid AB, A2+ has approximately the same radius as Li+, and B2- has approximately the same radius as F?. What is a reasonable estimate of the lattice energy of AB?
A) About 3 × (-1033 kJ/mol)
B) About -1033 kJ/mol
C) About 4 × (-1033 kJ/mol)
D) About 2 × (-1033 kJ/mol)
E) About 6 × (-1033 kJ/mol)
Correct Answer

verified
Correct Answer
verified
Short Answer
Use the data to calculate the lattice energy of sodium chloride. 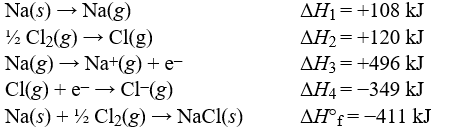
Correct Answer

verified
Correct Answer
verified
True/False
The lattice energy of Al2O3 is greater than that for AlCl3, which implies that the melting point of Al2O3 should be greater than that of AlCl3.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for HClO3. If the valence shells are filled to the usual limit (a maximum of 8) , what is the formal charge on the chlorine atom? Hint: Do not forget about double or triple bonds potentially being in the Lewis structure.
A) -1
B) 0
C) +1
D) +2
E) +3
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis dot symbol for the S−2 ion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete the Lewis structures for COCl2 and SOCl2 using the skeletal structure shown below, being sure to follow the procedure for minimizing the sum of the absolute values for the formal charges where the octet rule need not be followed. Based on the complete structures, which statement below is true?  Hint: Consider the potential for multiple bonds on the molecules and be sure to account for all valence electrons.
Hint: Consider the potential for multiple bonds on the molecules and be sure to account for all valence electrons.
A) The COCl2 exhibits formal charges but no resonance hybrids, while the SOCl2 exhibits both formal charges and resonance hybrids.
B) The COCl2 exhibits both residual formal charges and resonance hybrids, while the SOCl2 exhibits formal charges but no resonance hybrids.
C) The SOCl2 exhibits formal charges but no resonance hybrids, while the COCl2 exhibits resonance hybrids but no formal charges.
D) The SOCl2 exhibits both formal charges and resonance hybrids, while the COCl2 exhibits resonance hybrids but no formal charges.
E) The formal charges on all the atoms are zero, and the molecules have no resonance hybrids.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond is the most polar?
A) H-C
B) H-S
C) H-P
D) H-O
E) H-Se
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is expected to produce an acidic solution when dissolved in water?
A) CH3CH2CH2CH3
B) CH3CH2OH
C) CH3COCH3
D) CH3NHCH3
E) CH3CH2CO2H
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is expected to produce a basic solution when dissolved in water?
A) CH3CH2CH2CH3
B) CH3CH2OH
C) CH3COCH3
D) CH3CH2NH2
E) CH3CH2CO2H
Correct Answer

verified
Correct Answer
verified
Short Answer
Use the data to calculate the lattice energy of sodium bromide. 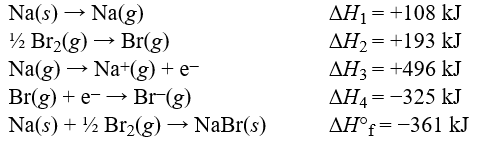
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atoms in the oxygen molecule, O2, are held together by Hint: Draw the Lewis structure of the molecule.
A) a single covalent bond.
B) a double covalent bond.
C) a triple covalent bond.
D) an ionic bond.
E) a magnetic dipole bond.
Correct Answer

verified
Correct Answer
verified
True/False
Compounds with negative heats of formation tend to be unstable with respect to decomposition into its elements.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Bromine tends to form a monatomic ion that has the electron configuration of a noble gas. What is the symbol of that noble gas?
A) Ne
B) Ar
C) Kr
D) Xe
E) Rn
Correct Answer

verified
Correct Answer
verified
Short Answer
The amount of energy released when a covalent bond is formed is called its ________.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 167
Related Exams