Correct Answer

verified
Correct Answer
verified
Short Answer
There are ________ sulfur atoms in 25.6 g of Al2(S2O3)3. Hint: You can't convert directly from mass of a compound to atoms of an element. Use an intermediary.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a chemical equation, C3H6O2 + PCl3 C3H5OCl + H3PO3, when the reaction was carried out, the actual yield of C3H5OCl was calculated as 97.3 % of the theoretical value. If the theoretical yield should have been 1.42 moles, how many grams of C3H5OCl were actually obtained?
A) 115 grams
B) 128 grams
C) 156 grams
D) 193 grams
E) 206 grams
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of Ni(CO) 4, a toxic transition-metal complex, has 5.23 × 1024 atoms of carbon. How many atoms of Ni does it contain?
A) 6.02 × 1023 atoms
B) 1.50 × 1023 atoms
C) 4.67 × 1022 atoms
D) 20.9 × 1023 atoms
E) 1.31 × 1024 atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic mass of aluminum is 26.982 u. How many aluminum atoms are in a 4.55 g sample of aluminum?
A) 1.02 × 1023
B) 1.32 × 1023
C) 2.74 × 1024
D) 3.57 × 1024
E) 8.01 × 1023
Correct Answer

verified
Correct Answer
verified
Short Answer
Given the chemical equation, 2Mg + O2 → 2MgO, when 2.2 g Mg react with 3.6 g of O2, 2.7 g MgO were obtained. What is the percent yield in the reaction?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound contains potassium, sulfur, and oxygen. The composition of the compound is: potassium, 49.410%; sulfur, 20.261%. Determine the empirical formula of the compound. Hint: Assume you have 100 grams of this substance and use moles to relate the mass of one element to another.
A) K2SO3
B) K2SO4
C) K2S2O4
D) K2S2O3
E) K3S2O8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reactants and products in a reaction are shown below in the unbalanced chemical equation: P4O10 + Ca(OH) 2 Ca3(PO4) 2 + H2O If you start with 2.40 moles of P4O10 and 3.30 moles of Ca(OH) 2 and all of the limiting reactant is consumed, Hint: You must balance the equation first.
A) 1.10 moles of Ca3(PO4) 2 are produced.
B) 0.90 moles of Ca(OH) 2 are left over.
C) 2.40 moles of Ca3(PO4) 2 are produced.
D) 1.65 moles of Ca3(PO4) 2 are produced.
E) 0.85 moles of P4O10 are consumed.
Correct Answer

verified
Correct Answer
verified
Short Answer
In the course of determination of a chemical formula, a student obtained the following mole ratios: 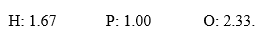 .The empirical formula for the compound is ________.
.The empirical formula for the compound is ________.
Correct Answer

verified
Correct Answer
verified
Short Answer
The Hall process for the production of aluminum involves the reaction of aluminum oxide with elemental carbon to give aluminum metal and carbon monoxide. If the yield of this reaction is 82% and aluminum ore is 71% by mass aluminum oxide, what mass of aluminum ore must be mined in order to produce 1.0 × 103 kg (1 metric ton)of aluminum metal by the Hall process? Hint: You need to first determine how product you need to aim for to accommodate an actual yield of 82%, and then account for the fact that the ore is only 71% aluminum oxide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic mass of helium is 4.0026 u. What is the mass of a helium sample that contains 0.427 moles of He gas?
A) 0.427 g
B) 0.107 g
C) 1.71 g
D) 2.57 g
E) 9.37 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You are given the balanced equation: C4H4 + 5 O2 4 CO2 + 2 H2O If 0.3618 moles of C4H4 are allowed to react with 1.818 moles of O2, and this is the only reaction which occurs, what is the maximum quantity of carbon dioxide that could be produced?
A) 1.447 moles
B) 1.454 moles
C) 1.456 moles
D) 2.180 moles
E) 0.3978 moles
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the expected yield of Pb, if 25.0 g C react with 25.0 g of PbO according to the chemical equation, PbO + C → CO + Pb?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 6.987 g sample of a hydrocarbon, upon combustion analysis, yielded 8.398 grams of carbon dioxide. What is the percent, by mass, of carbon in the hydrocarbon?
A) 16.01 %
B) 23.66 %
C) 32.80 %
D) 34.91 %
E) 58.68 %
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound has an empirical formula of CH2O. An independent analysis gave a value of 150.13 g for its molar mass. What is the molecular formula of the compound?
A) C5H10O5
B) C6H12O6
C) C11H2O
D) C6H6O8
E) C9H10O2
Correct Answer

verified
Correct Answer
verified
True/False
A mole of oxygen, O2, and a mole of ozone, O3, contain the same number of molecules.
Correct Answer

verified
Correct Answer
verified
Short Answer
A laboratory unknown is a mixture of calcium carbonate and magnesium carbonate. These substances decompose to form calcium oxide and magnesium oxide, respectively, when heated at a high temperature for a prolonged period. A sample of this laboratory unknown mixture with a mass of 5.424 grams was decomposed by heating for several hours at 950 °C. The oxide residue produced had a mass of 2.791 grams. From this laboratory data calculate the percent, by weight, of calcium carbonate in the unknown. Hint: Use the balanced chemical equations to find the molar ratios of the carbonates to the oxides, then create two algebraic equations - one for the carbonates and the other for the products. This will give you two equations with two unknowns, which can be solved to determine the number of moles of one of your substances.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A freshly prepared compound contains potassium, hydrogen, phosphorus, and oxygen. The composition of the compound is: potassium, 44.895%; hydrogen, 0.5787%; phosphorus, 17.783%. Determine the empirical formula of the compound. Hint: Assume you have 100 grams of this substance and use moles to relate the mass of one element to another.
A) K2H4P4O
B) K2HPO4
C) K2H2PO4
D) K2HP2O5
E) KH2PO4
Correct Answer

verified
Correct Answer
verified
Short Answer
The mass of 3.00 moles of phosphorus (P4), is ________ grams.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the percent, by mass, of calcium in Ca(OCl) 2?
A) 28.030%
B) 28.571%
C) 31.562%
D) 43.787%
E) 44.493%
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 207
Related Exams