A) a fuel that will spontaneously decay via a radioactive process.
B) a moderator component to absorb neutrons and control the rate of the nuclear reactions.
C) a steam generator/turbine component that is fundamentally different than that found in coal driven power plants.
D) enriched grade uranium fuel.
E) a control component to limit the number neutrons available to trigger fission reactions.
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Why do fission products tend to be radioactive?
A) They have excess electrons.
B) They have excess protons.
C) The neutron to proton ratio is high.
D) The neutron to proton ratio is low.
E) They emit gamma rays.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nuclide with the formula 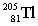 is best described as
is best described as
A) stable.
B) containing 81 neutrons and having a mass number of 205.
C) containing 205 protons and 81 neutrons.
D) containing 81 neutrons and 124 protons.
E) containing 124 neutrons and 81 protons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Melvin Calvin received a Noble Prize for unravelling the reaction sequence of the production of sugar in plants. How was he able to do this?
A) using tritium to slow the reactions down
B) taking multiple x-rays as a plant is growing
C) using a radioactive tracer to follow the carbon throughout the plant
D) growing a plant by only using human breath
E) using Carbon-14 dating
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a characteristic of fission reactions?
A) Only a few nuclides undergo fission.
B) Fission produces large amounts of energy.
C) Fission gives only a few nuclides as products.
D) Fission products are often radioactive.
E) Fission products always include one or more neutrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Shielding is an important aspect of working with radioactive materials. Which of the following is the order of radiation type in order of increasing penetration?
A) , ,
B) ,
C) , ,
D) , ,
E) , ,
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the binding energy per mole of nucleons for the boron isotope with mass of 12.0143 amu.(If needed, use the following equations: E = mc2 c = 3.0 x 108m/s E = m(8.988 x 1010 kJ/g) massproton = 1.007276 g/mol massneutron = 1.008665 g/mol masselectron = 0.0005486)
A) -1.537 x 109 kJ/mol
B) -6.404 x 108 kJ/mol
C) 6.404 x 108 kJ/mol
D) 1.736 x 1010 kJ/mol
E) -7.685 x 108 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nuclei that lie below the belt of stability generally decay by
A) emission.
B) emission.
C) electron capture.
D) x-ray emission.
E) emission.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nuclei that lie above the belt of stability generally decay by
A) emission.
B) emission.
C) electron capture.
D) positron emission.
E) emission.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which unstable species decays with the emission of an alpha and a gamma particle to form 222Rn?(If needed, use the following equation: 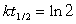
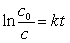 )
)
A) 226Po
B) 226Fr
C) 226Ra
D) 224Ra
E) 224Po
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a fusion reaction?
A) 1H + 1H 2He +
B) 1H + 12C 14N
C) 13C + 4He 16O + 1n
D) 235U + 1n 153Nd + 81Ge + 3 1n
E) 56Fe + 1n 57Co +
Correct Answer

verified
Correct Answer
verified
Multiple Choice
64Ni and a positron are formed when which unstable species decays?(If needed, use the following equation: 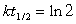
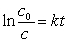 )
)
A) 64Co
B) 64Cu
C) 65Co
D) 65Cu
E) 63Co
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are differences between a nuclear reactor and a nuclear fission weapon?
A) The neutron recapture ratio.
B) The type of fuel.
C) The method of concentrating the fuel.
D) b and c
E) The kind of nuclear reaction occurring.
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
A cyclotron could not be used to accelerate which of the following particles?
A) electron
B) proton
C) particle
D) neutron
E) 11B5+
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Positron emission and electron capture cannot be directly observed, but are indirectly observed by the
A) emission of visible light.
B) capture of the particles.
C) observation of high-energy electromagnetic radiation.
D) change in mass of the nuclide.
E) increase in half-life of the daughter nuclide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nuclide with the formula 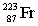 is best described as
is best described as
A) containing 136 neutrons and 87 protons.
B) containing 87 neutrons and having a mass number of 223.
C) containing 223 electrons and 87 protons.
D) containing 87 neutrons and 136 protons.
E) stable.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Neutron bombardment of nuclei typically requires lower energies than proton bombardment of nuclei. The energy difference is because
A) the strong nuclear force has a stronger attraction for the neutron.
B) gravity acts more strongly on the neutron.
C) the proton is attracted to the electron cloud.
D) the proton is repelled by the nucleus.
E) the electron cloud gives the proton a spiraling approach.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radiation treatment for cancer is effective because
A) cancer cells absorb more radiation than normal cells.
B) radioactive isotopes bind to tumours.
C) radiation has deleterious effects on rapidly dividing cells.
D) radiation can be precisely focused on cancerous cells.
E) it is less costly than other treatments.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the primary cause of biological damage from radioactive emissions?
A) genetic mutation
B) loss of body fluids
C) sunburn from hair loss
D) ionization of molecules
E) opportunistic infection
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The main impediment to fusion
A) is the fact that these are first-order reactions, so they are kinetically slow.
B) fusion occurs only for a few very rare nuclides.
C) reacting nuclei must have high kinetic energies to penetrate electron clouds.
D) the fusion products are often radioactive.
E) reacting nuclei must have high kinetic energies to overcome electrical repulsion between positive particles.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 44
Related Exams