Correct Answer

verified
oxidation: 2 Fe → 2 ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
During the recharging of a lead storage battery, what products(s) are formed?
A) PbO2 (s) and PbSO4 (s)
B) Pb (s) and PbO2 (s)
C) Pb (s) only
D) PbO2 (s) only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a correctly balanced oxidation half-reaction?
A) H2C2O4 2 CO2 + 2 H+ + 2e?
B) I2 + 2e- 2 I-
C) HOCl + H+ + 2e- Cl- + H2O
D) H2S S + 2 H+ + 4e-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate whether each of the following formula unit or net ionic equations is or is not a redox reaction by writing the word -2 Al(s) + 3 FeO(s) 3 Fe(s) + Al2O3(s) ____________________
A) non-redox
B) redox
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a galvanic cell, the cathode is the electrode at which ________ occurs?
A) oxidation
B) reduction
C) oxidation and reduction
D) nothing
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the oxidation number of the underlined element in NaBrO4.
A) +7
B) +4
C) -6
D) -4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following reaction: 2 Al (s) + 3 I2 (s) 2 AlI3 (s) which species is the oxidizing agent?
A) Al
B) AlI3
C) I2
D) none of the species
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are lost or gained by each formula unit of CuBr2 in the reaction Zn + CuBr2 ZnBr2 + Cu
A) gains 2 electrons
B) loses 2 electrons
C) loses 1 electron
D) gains 6 electrons
Correct Answer

verified
Correct Answer
verified
Essay
Identify which substance is oxidized and which substance is reduced in the following chemical reaction. 4 NH3 + 3 O2 → 2 N2 + 6 H2O
Correct Answer

verified
NH3 is oxid...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
When the redox reaction: NF3 + AlCl3 N2 + Cl2 + AlF3 is balanced, the correct coefficients for NF3 and Cl2, respectively, are ________.
A) 3 and 4
B) 4 and 2
C) 2 and 3
D) 6 and 3
Correct Answer

verified
Correct Answer
verified
Essay
Balance the following equation by the oxidation-number method. Fe2O3 + CO → Fe + CO2
Correct Answer

verified
Fe2O3 + 3 CO...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following statements is not true of a redox reaction?
A) The reducing agent is the substance oxidized.
B) The reducing agent gains electrons.
C) The substance oxidized loses electrons.
D) The oxidizing agent is the substance reduced.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following reaction H2SO4 is ________. H2SO4 + HI I2 + SO2 + H2O
A) the oxidizing agent and is oxidized
B) the oxidizing agent and is reduced
C) the reducing agent and is oxidized
D) the reducing agent and is reduced
Correct Answer

verified
Correct Answer
verified
Essay
Assign an oxidation number to each atom in the following chemical reaction. As2O3 + 5 H2O + 2 I2 ? 2 H3AsO4 + 4 HI
Correct Answer

verified
Correct Answer
verified
Essay
Balance the following reactions for the solution type indicted. Be sure to identify which is oxidized and reduced.
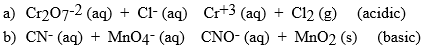
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following equations is incorrectly classified as to type of chemical reaction?
A) CaCO3 CaO + CO2 decomposition/redox
B) H2O + SO2 H2SO3 synthesis/nonredox
C) Cl2 + F2 2 ClF synthesis/redox
D) AgNO3 + NaCl AgCl + NaNO3 double-displacement/non-redox
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The proper assignment of oxidation numbers to the elements in Na2CrO4 would be ________.
A) +1 for Na, +6 for Cr and -2 for O
B) +2 for Na, +4 for Cr and -6 for O
C) +2 for Na, +3 for Cr and -2 for O
D) +2 for Na, +5 for Cr and -6 for O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reactions below, which reaction correctly identifies the anode reaction during the discharging (normal usage) of a lead storage battery cell?
A) Pb + SO42- PbSO4 + 2 e-
B) Zn + 2 OH- ZnO + H2O + 2 e-
C) 2 MnO2 + H2O + 2 e- Mn2O3 + 2 OH-
D) PbO2 + 4 H+ + SO42- + 2 e- PbSO4 + 2 H2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate whether each of the following formula unit or net ionic equations is or is not a redox reaction by writing the word -N2(g) + 3 H2(g) 2 NH3(g) ____________________
A) non-redox
B) redox
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which reaction is the correctly balanced half reaction (in acid solution) for the process below? Cr2O72- (aq) Cr3+ (aq)
A) 14 H+ + Cr2O72- + 6 e- 2 Cr3+ + 7 H2O
B) 8 H+ + Cr2O7 + 3 e- 2Cr3+ + 4 H2O
C) 8 H+ + Cr2O7 2 Cr3+ + 4 H2O + 3 e-
D) 12 H+ + Cr2O72- + 3 e- 2 Cr3+ + 6 H2O
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 70
Related Exams