A) has a negative charge < -1
B) contains both a metal and a nonmetal
C) develops a charge as a result of the combination of two or more types of atoms
D) occurs alone as do molecules
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula for the ionic compound which forms when Co3+ combines with SO42-?
A) CoSO4
B) Co2(SO4) 3
C) Co(SO4) 2
D) Co3(SO4) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules contains a triple covalent bond?
A) Br2
B) C2H2Cl2
C) SO2
D) HCN
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate the total number of electrons which would be shown as "dots" in a correctly written Lewis structure for OF2.
A) 18
B) 32
C) 26
D) 20
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structures for the following compounds. Draw all possible resonance structures. What is the molecular geometry of each compound? -NO3-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When drawing a Lewis structure, pairs of electrons that are not between atoms but are used to fill the octet of an atom are called ________.
A) bonding pairs
B) lone pairs
C) filled shells
D) excess electrons
Correct Answer

verified
Correct Answer
verified
Short Answer
How many valence electrons do atoms with the following electron configurations have? -1s22s22p5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct formula for the ionic compound containing Ni2+ and NO3? ions is ________.
A) NiNO3
B) Ni2NO3
C) Ni(NO3) 3
D) Ni(NO3) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The geometry associated with three pairs of bonded electrons and one pair of nonbonded electrons about a central atom in a molecule is ________.
A) tetrahedral
B) trigonal pyramidal
C) trigonal planar
D) angular
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Four hypothetical elements, A, B, C and D, have the following electronegativities and periodic table positions:
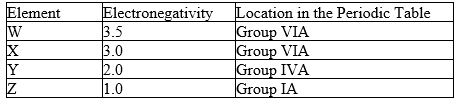 -The compound formed between W and Y would have:
-The compound formed between W and Y would have:
A) ionic bonds
B) polar covalent bonds
C) nonpolar covalent bonds
D) both ionic and polar covalent bonds
Correct Answer

verified
Correct Answer
verified
Short Answer
How many valence electrons do atoms with the following electron configurations have? -1s22s22p63s23p64s1
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structure for formic acid, HCOOH, in the order given.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds contains an ion with a 3+ charge?
A) KCl
B) AlP
C) BeF2
D) BaO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Four hypothetical elements, A, B, C and D, have the following electronegativities and periodic table positions:
 -The formula of the compound between X and Z would be:
-The formula of the compound between X and Z would be:
A) ZX
B) XZ
C) XZ2
D) Z2X
E) ZX3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct Lewis symbol diagram for carbon?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a nonpolar molecule containing polar bonds?
A) H - H
B) CH4
C) F - Be - F
D) It is not possible for a molecule to be nonpolar while containing polar bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The notation Y3- denotes a Y atom that has ________.
A) lost 3 electrons
B) lost 3 protons
C) gained 3 electrons
D) gained 3 protons
Correct Answer

verified
Correct Answer
verified
Short Answer
Determine the formula for the compound formed between the following pairs of elements and/or ions. -Cs and P4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ability of an atom to attract electrons to itself in a molecule is called ________.
A) electron affinity
B) electronegativity
C) ionization energy
D) paramagnetism
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bonding is not possible for carbon with its 4 valence electrons?
A) 4 single bonds
B) 2 single bonds and 1 double bond
C) 1 single bond and 1 triple bond
D) 1 double bond and 1 triple bond
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 92
Related Exams