Correct Answer

verified
Correct Answer
verified
Short Answer
The following questions refer to the functional groups shown in Figure 4.6.
A.
D.
B.
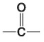 E. - SH
C.
E. - SH
C.
 -Which is an acidic functional group that can dissociate and release H+ into a solution?
-Which is an acidic functional group that can dissociate and release H+ into a solution?
Correct Answer

verified
Correct Answer
verified
Short Answer
The following questions refer to the functional groups shown in Figure 4.6.
A.
D.
B.
 E. - SH
C.
E. - SH
C.
 -Which is a carboxyl functional group?
-Which is a carboxyl functional group?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemist wishes to make an organic molecule less acidic. Which of the following functional groups should be added to the molecule in order to do so?
A) carboxyl
B) sulfhydryl
C) hydroxyl
D) amino
E) phosphate
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound contains hydroxyl groups as its predominant functional group. Which of the following statements is True concerning this compound?
A) It lacks an asymmetric carbon, and it is probably a fat or lipid.
B) It should dissolve in water.
C) It should dissolve in a nonpolar solvent.
D) It won't form hydrogen bonds with water.
E) It is hydrophobic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Amino acids are acids because they always possess which functional group?
A) amino
B) carbonyl
C) carboxyl
D) sulfhydryl
E) aldehyde
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Thalidomide and L-dopa, shown below, are examples of pharmaceutical drugs that occur as enantiomers, or molecules that 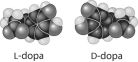
A) have identical three-dimensional shapes.
B) are mirror images of one another.
C) lack an asymmetric carbon.
D) differ in the location of their double bonds.
E) differ in their electrical charge.
Correct Answer

verified
Correct Answer
verified
Short Answer
The following questions refer to the molecules shown in Figure 4.7.
A.
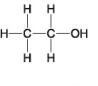 B.
B.
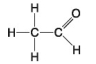 C.
C.
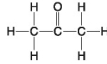 D.
D.
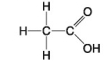 E.
E.
 -Which molecule has a carbonyl functional group in the form of an aldehyde?
-Which molecule has a carbonyl functional group in the form of an aldehyde?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the term that correctly describes the relationship between these two sugar molecules: 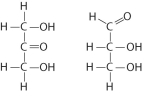
A) structural isomers
B) geometric isomers
C) enantiomers
D) isotopes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a False statement concerning amino groups?
A) They are basic in pH.
B) They are found in amino acids.
C) They contain nitrogen.
D) They are nonpolar.
E) They are components of urea.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Figure 4.4 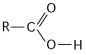 -What is the name of the functional group shown in Figure 4.4?
-What is the name of the functional group shown in Figure 4.4?
A) carbonyl
B) ketone
C) aldehyde
D) carboxyl
E) hydroxyl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why are hydrocarbons insoluble in water?
A) The majority of their bonds are polar covalent carbon-to-hydrogen linkages.
B) The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
C) They are hydrophilic.
D) They exhibit considerable molecular complexity and diversity.
E) They are lighter than water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following questions refer to the molecules shown in Figure 4.8.
A.
 B.
B.
 C.
C.
 D.
D.
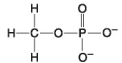 E.
E.
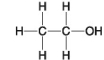 -Which of the following hydrocarbons has a double bond in its carbon skeleton?
-Which of the following hydrocarbons has a double bond in its carbon skeleton?
A) C?H?
B) C?H?
C) CH?
D) C?H?
E) C?H?
Correct Answer

verified
Correct Answer
verified
Short Answer
The following questions refer to the molecules shown in Figure 4.7.
A.
 B.
B.
 C.
C.
 D.
D.
 E.
E.
 -Which molecule is water soluble because it has a hydroxyl functional group?
-Which molecule is water soluble because it has a hydroxyl functional group?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
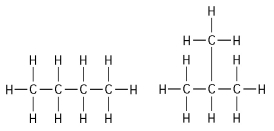 -The two molecules shown in Figure 4.1 are best described as
-The two molecules shown in Figure 4.1 are best described as
A) optical isomers.
B) radioactive isotopes.
C) structural isomers.
D) nonradioactive isotopes.
E) geometric isomers.
Correct Answer

verified
Correct Answer
verified
Short Answer
The following questions refer to the molecules shown in Figure 4.8.
A.
 B.
B.
 C.
C.
 D.
D.
 E.
E.
 -Which molecule functions to transfer energy between organic molecules?
-Which molecule functions to transfer energy between organic molecules?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many structural isomers are possible for a substance having the molecular formula C₄H₁₀?
A) 1
B) 2
C) 4
D) 3
E) 11
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electron pairs does carbon share in order to complete its valence shell?
A) 1
B) 2
C) 3
D) 4
E) 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
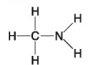
Which functional group is not present in this molecule?
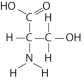
A) carboxyl
B) sulfhydryl
C) hydroxyl
D) amino
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following questions refer to the molecules shown in Figure 4.8.
A.
 B.
B.
 C.
C.
 D.
D.
 E.
E.
 -Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other?
-Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other?
A) Testosterone and estradiol are structural isomers but have the same molecular formula.
B) Testosterone and estradiol are geometric isomers but have the same molecular formula.
C) Testosterone and estradiol have different functional groups attached to the same carbon skeleton.
D) Testosterone and estradiol have distinctly different chemical structures, with one including four fused rings of carbon atoms, while the other has three rings.
E) Testosterone and estradiol are enantiomers of the same organic molecule.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 68
Related Exams