A) Only D will form hydrogen bonds with water.
B) All of these molecules will form hydrogen bonds with water.
C) None of these molecules will form hydrogen bonds with water.
D) All of these molecules except B will form hydrogen bonds with water.
E) Only C, D, and E will form hydrogen bonds with water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A carbon skeleton is covalently bonded to both an amino group and a carboxyl group. When placed in water, it
A) would function only as an acid because of the carboxyl group.
B) would function only as a base because of the amino group.
C) would function as neither an acid nor a base.
D) would function as both an acid and a base.
E) is impossible to determine how it would function.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
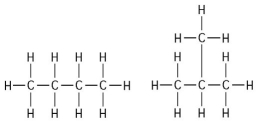 -The two molecules shown in the figure above are best described as
-The two molecules shown in the figure above are best described as
A) optical isomers.
B) enantiomers.
C) structural isomers.
D) cis-trans isomers.
E) chain length isomers.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You are investigating the differences between three organic compounds. They all have the same carbon skeleton but have differences in their functional groups. -Which chemical group is most likely to be responsible for an organic molecule behaving as a base?
A) hydroxyl
B) carbonyl
C) carboxyl
D) amino
E) phosphate
Correct Answer

verified
Correct Answer
verified
Multiple Choice
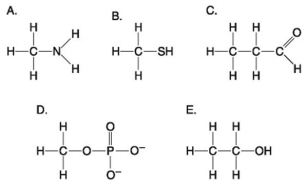
Answer the following questions based on the figure below.
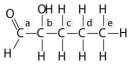 -Which action could produce a carbonyl group?
-Which action could produce a carbonyl group?
A) the replacement of the -OH of a carboxyl group with hydrogen
B) the addition of a thiol to a hydroxyl
C) the addition of a hydroxyl to a phosphate
D) the replacement of the nitrogen of an amine with oxygen
E) the addition of a sulfhydryl to a carboxyl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
 -A chemist wishes to make an organic molecule less acidic. Which of the following functional groups should be added to the molecule in order to do so?
-A chemist wishes to make an organic molecule less acidic. Which of the following functional groups should be added to the molecule in order to do so?
A) carboxyl
B) sulfhydryl
C) hydroxyl
D) amino
E) phosphate
Correct Answer

verified
Correct Answer
verified
Multiple Choice
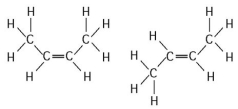 -The two molecules shown in the figure above are best described as
-The two molecules shown in the figure above are best described as
A) enantiomers.
B) radioactive isotopes.
C) structural isomers.
D) nonisotopic isomers.
E) cis-trans isomers.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When Stanley Miller applied heat and electrical sparks to a mixture of simple inorganic compounds such as methane, hydrogen gas, ammonia, and water vapour, what compounds were produced?
A) mostly amino acids
B) only simple organic compounds such as formaldehyde and cyanide
C) mostly hydrocarbons
D) only simple inorganic compounds
E) both simple organic compounds and more complex organic compounds such as amino acids and hydrocarbons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many structural isomers are possible for a substance having the molecular formula C₄H₁₀?
A) 1
B) 2
C) 4
D) 3
E) 11
Correct Answer

verified
Correct Answer
verified
Multiple Choice
L-dopa and D-dopa, shown below, are an example of a pharmaceutical drug that occurs as enantiomers, or molecules that 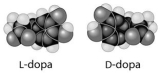
A) have identical three-dimensional shapes.
B) are mirror images of one another.
C) are structural isomers.
D) are mirror images of one another and have the same biological activity.
E) are cis-trans isomers.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements correctly describes cis-trans isomers?
A) They have variations in arrangement around a double bond.
B) They have an asymmetric carbon that makes them mirror images.
C) They have the same chemical properties.
D) They have different molecular formulas.
E) Their atoms and bonds are arranged in different sequences.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
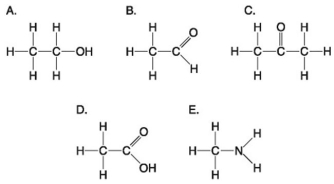 -Which molecules shown above contain a carbonyl group?
-Which molecules shown above contain a carbonyl group?
A) A and B
B) B and C
C) B, C, and D
D) D and E
E) E and A
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
 -Which molecule shown above can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
-Which molecule shown above can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound contains hydroxyl groups as its predominant functional group. Which of the following statements is true concerning this compound?
A) It lacks an asymmetric carbon, and it is probably a fat or lipid.
B) It should dissolve in water.
C) It should dissolve in a nonpolar solvent.
D) It won't form hydrogen bonds with water.
E) It is hydrophobic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers, that is, molecules that
A) have identical chemical formulas but differ in the branching of their carbon skeletons.
B) are mirror images of one another.
C) exist in either linear chain or ring forms.
D) differ in the location of their double bonds.
E) differ in the arrangement of atoms around their double bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
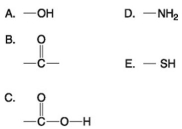 -Which functional group shown above is characteristic of alcohols?
-Which functional group shown above is characteristic of alcohols?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The experimental approach taken in current biological investigations presumes that
A) simple organic compounds can be synthesized in the laboratory from inorganic precursors, but complex organic compounds like carbohydrates and proteins can only be synthesized by living organisms.
B) a life force ultimately controls the activities of living organisms and this life force cannot be studied by physical or chemical methods.
C) although a life force, or vitalism, exists in living organisms, this life force cannot be studied by physical or chemical methods.
D) living organisms are composed of the same elements present in nonliving things, plus a few special trace elements found only in living organisms or their products.
E) living organisms can be understood in terms of the same physical and chemical laws that can be used to explain all natural phenomena.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You are investigating the differences between three organic compounds. They all have the same carbon skeleton but have differences in their functional groups. -The second molecule is hydrophobic and has acidic properties. It most likely has
A) no functional groups attached and is a hydrocarbon.
B) at least one carboxyl group.
C) at least one amino group.
D) at least one carbonyl group.
E) phosphates.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The figure above shows the structures of glucose and fructose. These two molecules are
A) geometric isotopes.
B) enantiomers.
C) cis-trans isomers.
D) structural isomers.
E) nonisotopic isomers.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
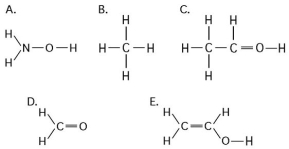 -Which of the structures illustrated above contain(s) a carbonyl functional group?
-Which of the structures illustrated above contain(s) a carbonyl functional group?
A) A
B) C and D
C) C
D) D
E) C and E
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 78
Related Exams