A) gain one
B) gain two
C) gain three
D) lose one
E) lose two
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What does "X" represent in the following symbol? 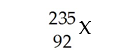
A) tin
B) copper
C) palladium
D) niobium
E) uranium
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What species is represented by the following information? p+ = 17 n° = 18 e- = 18
A) Cl
B) Cl-
C) Ar
D) Ar+
E) Kr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many silver atoms are contained in 3.75 moles of silver?
A) 6.23 × 1024 silver atoms
B) 2.26 × 1024 silver atoms
C) 1.61 × 1023 silver atoms
D) 2.44 × 1026 silver atoms
E) 6.50 × 1025 silver atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of Kr are contained in 398 mg of Kr?
A) 4.75 × 10-3 moles Kr
B) 33.4 moles Kr
C) 2.11 × 10-4 moles Kr
D) 2.99 × 10-3 moles Kr
E) 1.19 × 10-4 moles Kr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is a noble gas?
A) Ar
B) Br
C) N
D) O
E) K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the atomic mass of element "X" if it has two naturally occurring isotopes with the following masses and natural abundances: X-45 44.8776 amu 32.88% X-47 46.9443 amu 67.12%
A) 46.26 amu
B) 45.91 amu
C) 46.34 amu
D) 46.84 amu
E) 44.99 amu
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass (in mg) does 2.63 moles of nickel have?
A) 44.8 mg
B) 2.23 × 104 mg
C) 129 mg
D) 3.56 × 105 mg
E) 1.54 × 105 mg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the chemical symbol for copper?
A) Co
B) Cr
C) Cu
D) C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons does the Al3+ ion possess?
A) 16
B) 10
C) 6
D) 0
E) 13
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following describes a metal?
A) Poor conductor of heat
B) Good conductor of electricity
C) Tends to gain electrons
D) Forms ionic compounds with group 18 elements
E) Found on the upper right corner of the periodic table
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is classified as a semimetal?
A) calcium
B) boron
C) fluorine
D) uranium
Correct Answer

verified
Correct Answer
verified
Short Answer
Give an example of a halogen.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following sets do all species have the same number of electrons?
A) F-, Ne, Mg2+
B) Ge, Se2-, Br-
C) K+, Rb+, Cs+
D) Br, Br-, Br+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ion has 8 protons, 9 neutrons, and 10 electrons. The symbol for the ion is ________.
A) 17O2-
B) 17O2+
C) 19F+
D) 19F-
E) 17Ne2+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What element is defined by the following information? p+ = 17 n° = 20 e- = 17
A) calcium
B) rubidium
C) chlorine
D) neon
E) oxygen
Correct Answer

verified
Correct Answer
verified
Essay
Why do the isotopes of the same element have the same atomic size?
Correct Answer

verified
Isotopes only differ in the nu...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What species is represented by the following information? p+ = 47 n° = 62 e- = 46
A) Ag+
B) Nd
C) Pd
D) Ag
E) Pd+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When forming an ion, fluorine, F, will ________ electron(s) .
A) gain one
B) gain two
C) gain three
D) lose one
E) lose two
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the atomic mass of chromium if chromium has four naturally occurring isotopes with the following masses and natural abundances: Cr-50 49.9461 amu 4.35% Cr-52 51.9405 amu 83.79% Cr-53 52.9407 amu 9.50% Cr-54 53.9389 amu 2.36%
A) 51.941 amu
B) 51.699 amu
C) 208.766 amu
D) 53.214 amu
E) 51.996 amu
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 167
Related Exams