Correct Answer

verified
Neutrons, produced b...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Determine the identity of the daughter nuclide from the positron emission of 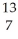 N.
N.
A) ![]() O
O
B) ![]() C
C
C) ![]() O
O
D) ![]() B
B
E) ![]() F
F
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the radioactive green light that glows in the dark.
A) phenolphthalein
B) radioactivity
C) phosphorescence
D) desensitivity
E) neon
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the alpha decay of 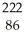 Rn.
Rn.
A) ![]() Po
Po
B) ![]() Ra
Ra
C) ![]() Th
Th
D) ![]() Rn
Rn
E) ![]() At
At
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the nuclear equation for the alpha decay of 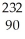 Th.
Th.
A) ![]() He +
He + ![]() Th →
Th → ![]() U
U
B) ![]() n +
n + ![]() Th →
Th → ![]() Th
Th
C) ![]() Th →
Th → ![]() e +
e + ![]() Ac
Ac
D) ![]() Th →
Th → ![]() He +
He + ![]() Ra
Ra
E) ![]() Th →
Th → ![]() e +
e + ![]() Pa
Pa
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents what nuclear process?  H +
H + 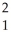 H →
H → 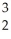 He +
He + 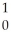 n
n
A) nuclear fusion
B) alpha emission
C) beta emission
D) nuclear fission
E) neutron capture
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atoms with Z > ________ are radioactive and decay in one or more steps involving mostly alpha and beta decay.
A) 60
B) 100
C) 83
D) 160
E) 40
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A freshly prepared sample of curium-243 undergoes 3312 disintegrations per second. After 6.00 y., the activity of the sample declines to 2755 disintegrations per second. The half-life of curium-243 is ________ y.
A) 4.99
B) 32.6
C) 7.21
D) 0.765
E) 22.6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the binding energy per nucleon of a Mg-24 nucleus. The Mg-24 nucleus has a mass of 24.30506. A proton has a mass of 1.00728 amu, a neutron has a mass of 1.008665 amu, and 1 amu is equivalent to 931 MeV of energy.
A) 0.3050 MeV
B) 8.83 MeV
C) 0.113 MeV
D) 106 MeV
E) 4.41 MeV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing particle in the following nuclear equation: 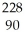 Th →
Th → 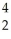 He + ?
He + ?
A) ![]() U
U
B) ![]() Ac
Ac
C) ![]() Ac
Ac
D) ![]() Ra
Ra
E) ![]() Ra
Ra
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing particle in the following nuclear equation: 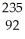 U →
U → 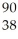 Sr + ? + 2
Sr + ? + 2 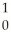 n + 4
n + 4 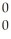 g
g
A) ![]() Te
Te
B) ![]() Xe
Xe
C) ![]() Xe
Xe
D) ![]() Te
Te
E) ![]() Sr
Sr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the beta decay of 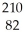 Pb.
Pb.
A) ![]() Pt
Pt
B) ![]() Tl
Tl
C) ![]() Hg
Hg
D) ![]() Bi
Bi
E) ![]() Pb
Pb
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing particle in the following nuclear equation: 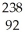 U → ? +
U → ? + 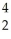 He + 2
He + 2 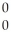 g
g
A) ![]() Th
Th
B) ![]() Ra
Ra
C) ![]() Pu
Pu
D) ![]() Th
Th
E) ![]() Ra
Ra
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the alpha decay of 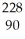 Th.
Th.
A) ![]() U
U
B) ![]() Pa
Pa
C) ![]() Ra
Ra
D) ![]() Ac
Ac
E) ![]() Th
Th
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents what nuclear process? 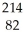 Pb →
Pb →  e +
e + 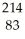 Bi
Bi
A) alpha emission
B) gamma emission
C) electron capture
D) neutron bombardment
E) beta emission
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which radiation has the highest penetrating power?
A) alpha rays
B) beta rays
C) gamma rays
D) positron emission
E) electron capture
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the half-life of a nuclide that loses 38.0% of its mass in 387 hours.
A) 277 hours
B) 455 hour
C) 561 hours
D) 639 hours
E) 748 hours
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of nucleons in a 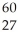 Co nucleus is
Co nucleus is
A) 27
B) 33
C) 60
D) 87
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which nuclide below is most likely to decay by electron capture?
A) ![]() Re
Re
B) ![]() Re
Re
C) ![]() Re
Re
D) ![]() Re
Re
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing particle in the following nuclear equation: 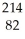 Pb →
Pb → 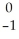 e + ?
e + ?
A) ![]() Bi
Bi
B) ![]() Tl
Tl
C) ![]() Pb
Pb
D) ![]() Pb
Pb
E) ![]() Tl
Tl
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 115
Related Exams