A) gasoline engine and an electric motor run by a rechargeable battery.
B) gasoline engine and a fuel cell.
C) fuel cell and an electric motor run by a rechargeable battery.
D) gasoline engine and a cleaner diesel engine.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is it so expensive to ship hydrogen as a liquid as is often done with other gas?
A) Hydrogen is very heavy to ship
B) Hydrogen is very dense to ship
C) Hydrogen has to be shipped at a very low temperature
D) Hydrogen in very flammable
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Reforming processes can store hydrogen fuel in a liquid form as another molecule.Which of the following cannot be reformed into hydrogen?
A) Methanol
B) Gasoline
C) Diesel
D) Carbon dioxide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following automotive energy storage devices would give rise to the fastest acceleration from zero to 60 mph?
A) Li-ion rechargeable batteries
B) Fuel cells
C) NiMH rechargeable batteries
D) Supercapacitors
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A NiCd battery uses nickel and cadmium to produce a potential difference.Using these equations,answer the following questions. I.2NiO(OH) (s) + 2H2O (l) + 2 e¯ 2Ni(OH) 2 (s) + 2 OH¯ (aq) II.Cd(s) + 2OH¯ (aq) Cd(OH) 2 (s) + 2e¯ III.Cd (s) + 2NiO(OH) (s) + 2 H2O (l) 2 Ni(OH) 2 (s) + Cd(OH) 2 (s) Which equation represents what takes place at the anode?
A) I
B) II
C) III
D) I and II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How do the interactions that are broken in water when it is boiled compare with those broken when water is electrolyzed?
A) Boiling water breaks intermolecular attractions and electrolysis breaks covalent bonds
B) Boiling water breaks covalent bonds and electrolysis breaks intermolecular attractions
C) Boiling water and electrolysis of water break covalent bonds
D) Boiling water and electrolysis of water break intermolecular forces
Correct Answer

verified
Correct Answer
verified
Multiple Choice
During the chemical reaction in an electrochemical cell,
A) a substance is oxidized and gains electrons.
B) electrons travel from the cathode to the anode.
C) oxidation takes place alone,without an accompanying reduction.
D) oxidation occurs at the anode.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why doesn't water naturally become hydrogen and oxygen gas when exposed to light?
A) Thermal energy is the only appropriate type of energy to split water
B) You must use electrons to split water
C) Water doesn't absorb the proper wavelength of light to split
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Batteries are still preferred over supercapacitors for portable electronic applications.What factor(s) give rise to this preference?
A) Supercapacitors have low energy densities
B) Supercapacitors are charged much more quickly than batteries
C) Supercapacitors have virtually unlimited cycle lives
D) All of these choices are correct
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does the reaction of hydrogen and oxygen to produce energy in a fuel cell differ from their interaction during the direct combustion of hydrogen and oxygen? 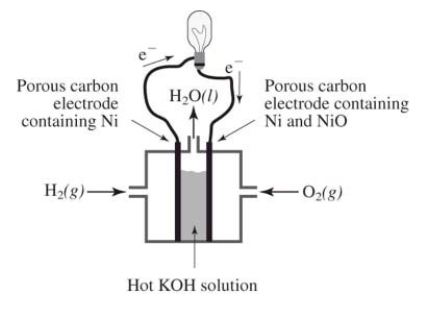 I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water
II) Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen
III) In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction
IV) It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell
I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water
II) Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen
III) In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction
IV) It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell
A) I and II only
B) I,II,and III only
C) I,II,III,and IV
D) I and III only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Whenever a substance is oxidized,
A) it is called the oxidizing agent.
B) some other substance must be reduced.
C) it gains electrons.
D) hydronium ions are produced.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the compound Fe3O4,what is the oxidation state of Fe?
A) 2+
B) 3+
C) 4+
D) Mixture between 2+ and 3+
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
In this electrochemical cell,the reduction half reaction is 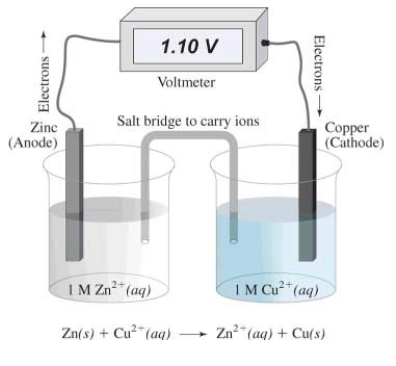
A) Cu2+(aq) + 2 e¯ Cu(s)
B) Zn(s) Zn2+(aq) + 2 e¯
C) Zn(s) Cu(s)
D) Cu2+(aq) Zn2+(aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Chemical energy is converted directly into electrical energy in
A) a galvanic cell.
B) an electrical power plant.
C) an electrolytic cell.
D) an automobile's engine.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the compound FeCl3,what is the oxidation state of Fe?
A) 2+
B) 3+
C) 4+
D) Mixture between 2+ and 3+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a fuel cell,
A) there is oxidation,but no reduction.
B) reduction takes place at the cathode.
C) there is direct conversion of mechanical energy into electricity.
D) a chemical reaction produces heat,which then produces electricity.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following statements is not true about PEM fuel cells?
A) PEM fuel cells rely on inexpensive catalysts
B) PEM fuels cells are cleaner than gasoline powered vehicles
C) PEM fuel cells have safe and efficient fuel storage
D) PEM fuel cells have limited driving range
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the typical units for specific energy?
A) mAh/g
B) W/kg
C) Wh/kg
D) kWh
Correct Answer

verified
C
Correct Answer
verified
Showing 1 - 18 of 18
Related Exams