A) 3.09 × 10-19 J
B) 6.14 × 10-19 J
C) 3.24 × 10-19 J
D) 1.63 × 10-19 J
E) 5.11 × 10-19 J
Correct Answer

verified
Correct Answer
verified
Essay
Why don't we observe the wavelength of everyday macroscopic objects?
Correct Answer

verified
Due to the large mass of macro...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Give all possible values of l for a n=5 sublevel.
A) 5
B) -1/2
C) 0, 1, 2, 3, 4
D) -3, -2, -1, 0, 1, 2, 3
E) 1, 2, 3, 4, 5
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Determine the mass of a ball with a velocity of 40.0 m/s and a wavelength of 8.92 × 10-34 m.
A) 29.7 g
B) 594 g
C) 2.36 g
D) 53.8 g
E) 18.6 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
________ are used to image bones and internal organs.
A) Ultraviolet light
B) Gamma rays
C) Microwaves
D) X-rays
E) Radio waves
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many photons are contained in a burst of yellow light (589 nm) from a sodium lamp that contains 616 kJ of energy?
A) 2.08 × 1013 photons
B) 3.06 × 1030 photons
C) 1.83 × 1024 photons
D) 4.03 × 1028 photons
E) 2.48 × 1025 photons
Correct Answer

verified
Correct Answer
verified
Essay
Give an example of a d orbital.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following types of electromagnetic radiation in order of increasing wavelength. ultraviolet light gamma rays microwaves
A) gamma rays < microwaves < ultraviolet light
B) microwaves < ultraviolet light < gamma rays
C) microwaves < gamma rays < ultraviolet light
D) ultraviolet light < gamma rays < microwaves
E) gamma rays < ultraviolet light < microwaves
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following transitions represent the emission of a photon with the largest energy?
A) n = 2 to n = 1
B) n = 3 to n = 1
C) n = 6 to n = 3
D) n = 1 to n = 4
E) n = 2 to n = 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following visible colors of light has the highest frequency?
A) green
B) red
C) blue
D) yellow
E) orange
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the wavelength (in nm) of a the red light emitted by a neon sign with a frequency of 5.00 × 1014 Hz.
A) 600 nm
B) 167 nm
C) 500 nm
D) 800 nm
E) 200 nm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the velocity of a marble (m = 7.75 g) with a wavelength of 3.46 × 10-33 m.
A) 40.5 m/s
B) 2.47 m/s
C) 24.7 m/s
D) 38.8 m/s
E) 52.9 m/s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the end (final) value of n in a hydrogen atom transition,if the electron starts in 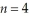 and the atom emits a photon of light with a wavelength of 486 nm.
and the atom emits a photon of light with a wavelength of 486 nm.
A) 1
B) 5
C) 3
D) 4
E) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the transition (in a hydrogen atom) below that represents the absorption of the shortest wavelength photon.
A) n = 1 to n = 2
B) n = 2 to n = 3
C) n = 4 to n = 6
D) n = 6 to n = 5
E) n = 3 to n = 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the energy of the red light emitted by a neon atom with a wavelength of 703.2 nm.
A) 3.54 × 10-19 J
B) 4.27 × 10-19 J
C) 2.34 × 10-19 J
D) 6.45 × 10-19 J
E) 2.83 × 10-19 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the numbers for ml for an f orbital.
A) 0, 1, 2, 3
B) 1, 2, 3, 4
C) -3, -2, -1, 0, 1, 2, 3
D) -2, -1, 0, 1, 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If two electrons in the same atom have the same value of "l",they are
A) in the same sublevel, but not necessarily in the same level.
B) in the same level, but different sublevel.
C) in the same orbital.
D) in different levels and in different shaped orbitals.
E) none of the above.
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.88 × 1014 Hz.
A) 229 nm
B) 436 nm
C) 206 nm
D) 485 nm
E) 675 nm
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
When a wave encounters an obstacle or a slit that is comparable in size to its wavelength,it bends around it.This characteristic is called
A) destructive interference.
B) diffraction.
C) constructive interference.
D) effusion.
E) amplitude.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a hydrogen atom,which electronic transition would result in the emission of a photon with the highest energy?
A) 1s → 2p
B) 3p → 7d
C) 3p → 1s
D) 6f → 4d
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 141
Related Exams