A) wavelength.
B) amplitude.
C) frequency.
D) area.
E) median.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
It is possible to determine the ionization energy for hydrogen using the Bohr equation.Calculate the ionization energy for an atom of hydrogen,making the assumption that ionization is the transition from 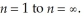
A) -2.18 × 10-18 J
B) +2.18 × 10-18 J
C) +4.59 × 10-18 J
D) -4.59 × 10-18 J
E) +4.36 × 10-18 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When waves of equal amplitude from two sources are out of phase when they interact,it is called
A) destructive interference.
B) diffraction.
C) constructive interference.
D) effusion.
E) amplitude.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An FM radio station broadcasts electromagnetic radiation at a frequency of 92.6 MHz.The wavelength of this radiation is ________ m.
A) 3.24 × ![]()
B) 3.24
C) 2.78 × ![]()
D) 2.78 × ![]()
E) 0.309
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following colors of electromagnetic radiation has the shortest wavelength?
A) blue
B) violet
C) orange
D) green
E) yellow
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the wavelength of an electron (m = 9.11 × 10-28 g) moving at 1/5 the speed of light?
A) 9.36 × 10-9 m
B) 1.21 × 10-11 m
C) 6.73 × 10-8 m
D) 2.42 × 10-12 m
E) 4.85 × 10-10 m
Correct Answer

verified
Correct Answer
verified
Matching
Match the following.
Correct Answer
Multiple Choice
Identify the color that has a wavelength of 545 nm.
A) blue
B) green
C) red
D) yellow
Correct Answer

verified
Correct Answer
verified
Short Answer
Define constructive interference.
Correct Answer

verified
Waves that...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Determine the mass of a ball with a wavelength of 3.45 × 10-34 m and a velocity of 6.55 m/s.
A) 0.293 g
B) 12.6 g
C) 293 g
D) 346 g
E) 3.41 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which orbital below would an electron (on average) be closest to the nucleus?
A) 2p
B) 4s
C) 2s
D) 5d
E) 3p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the frequency of the light emitted a photon with a wavelength of 120 nm.
A) 1.03 × 1015 s-1
B) 2.50 × 1015 s-1
C) 5.38 × 1015s-1
D) 2.36 × 1015 s-1
E) 7.42 × 1015 s-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many orbitals are present when l=3?
A) 1
B) 3
C) 5
D) 7
E) 9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For hydrogen,what is the wavelength of the photon emitted when an electron drops from a 4d orbital to a 2s orbital in a hydrogen atom? The Rydberg constant is 1.097 × 10-2 nm-1.
A) 656.3 nm
B) 486.2 nm
C) 364.6 nm
D) 2.057 × 10-3 nm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many nodes are found in a 3s orbital?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following types of waves are considered electromagnetic radiation: A.Radio Waves; B.Sound Waves; C.Microwaves; D.Ocean Waves; E.Mechanical Waves
A) A and B
B) A and C
C) B and D
D) A,C,and E
E) B,C,and D
Correct Answer

verified
Correct Answer
verified
Essay
Why do atoms only emit certain wavelengths of light when they are excited? (Why do line spectra exist?)
Correct Answer

verified
The energies of atoms are quantized.When...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Calculate the frequency of the green light emitted by a hydrogen atom with a wavelength of 486.1 nm.
A) 1.46 × 1014 s-1
B) 6.86 × 1014 s-1
C) 4.33 × 1014 s-1
D) 6.17 × 1014 s-1
E) 1.62 × 1014 s-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of f orbitals that are possible in a given shell?
A) 1
B) 3
C) 7
D) 5
E) 9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the possible values for ml for a d orbital.
A) 0, 1, 2
B) -1, 0, 1
C) 1, 2, 3
D) -2, -1, 0, 1, 2
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 141
Related Exams