A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for the molecule CH2CHCH3.How many sigma and pi bonds does it contain?
A) 8 sigma, 1 pi
B) 9 sigma, 0 pi
C) 9 sigma, 1 pi
D) 7 sigma, 2 pi
E) 8 sigma, 2 pi
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the number of electron groups around a molecule with sp2 hybridization.
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
A) B22⁺
B) B22⁻
C) N22⁺
D) C22⁻
E) B2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule containing a central atom with sp3 hybridization has a(n) ________ electron geometry.
A) linear
B) trigonal bipyramidal
C) octahedral
D) tetrahedral
E) bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the molecular orbital diagram shown to determine which of the following is MOST stable.
A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B22⁺
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules have sp2 hybridization on the central atom? HCN SO2 OCl2 XeCl2
A) 4
B) 3
C) 2
D) 1
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the hybridization for the C in C2F2.
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the hybridization for the Br in BrCl3.
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp
Correct Answer

verified
Correct Answer
verified
Essay
Determine the hybridization about each interior atom in the following structure.Sketch the three-dimensional structure and label the interior atoms with their corresponding hybridization. CH2CHCCCH3
Correct Answer

verified
The sketch should show all of the approp...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Give the hybridization for the S in SO3.
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Draw the molecular orbital diagram shown to determine which of the following is MOST stable.
A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F22⁻
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
List the number of sigma bonds and pi bonds in a single bond.
A) 1 sigma, 0 pi
B) 0 sigma, 1 pi
C) 1 sigma, 1 pi
D) 3 sigma, 2 pi
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
A) O22⁻
B) Ne22⁺
C) O22⁺
D) F22⁺
E) None of the above are paramagnetic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the electron geometry (eg) ,molecular geometry (mg) ,and hybridization for POCl3.
A) eg = tetrahedral, mg = tetrahedral, sp3
B) eg = trigonal pyramidal, mg = trigonal pyramidal, sp3
C) eg = octahedral, mg = square planar, sp3d2
D) eg = octahedral, mg = octahedral, sp3d2
E) eg = trigonal bipyramidal, mg = seesaw, sp3d
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Give the electron geometry (eg) ,molecular geometry (mg) ,and hybridization for H2O.
A) eg = tetrahedral, mg = bent, sp3
B) eg = trigonal pyramidal, mg = trigonal pyramidal, sp3
C) eg = tetrahedral, mg = trigonal pyramidal, sp3
D) eg = bent, mg = bent, sp2
E) eg = trigonal planar, mg = trigonal planar, sp2
Correct Answer

verified
Correct Answer
verified
Matching
Match the following.
Correct Answer
Essay
Use molecular orbital theory to determine whether He22⁺ or He2⁺ is more stable.Draw the molecular orbital diagram for each and explain your answer.
Correct Answer

verified
The MO diagram should show He22⁺ with 2 el...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is MOST stable. 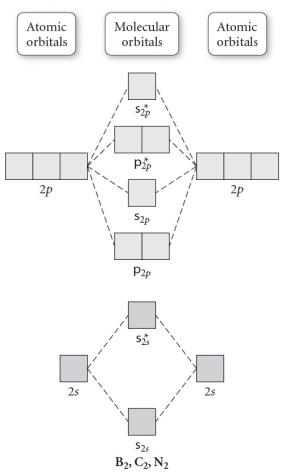
A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B22⁺
Correct Answer

verified
Correct Answer
verified
Essay
Give the electron geometry,molecular geometry,and hybridization for both carbons in CH3COOH.
Correct Answer

verified
eg = tetrahedral; mg...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 1 - 20 of 64
Related Exams