A) 2.92 × 104 years
B) 1.94 × 104 years
C) 8.92 × 103 years
D) 5.31 × 103 years
E) 1.74 × 102 years
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the nuclear equation for the alpha decay of 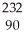 Th.
Th.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the positron emission of 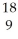 F.
F.
A) ![]() Na
Na
B) ![]() F
F
C) ![]() N
N
D) ![]() O
O
E) ![]() Ne
Ne
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Why is an alpha emitter much more harmful if ingested?
Correct Answer

verified
Alpha emitters have the highes...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Determine the identity of the daughter nuclide from the beta decay of 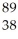 Sr.
Sr.
A) ![]() Sr
Sr
B) ![]() Y
Y
C) ![]() Y
Y
D) ![]() Kr
Kr
E) ![]() Se
Se
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nuclides above the valley of stability can become more stable through which of the following processes?
A) beta emission
B) positron emission
C) alpha emission
D) electron capture
E) neutron bombardment
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the following diagnostic procedure that gives the lowest dose of radiation.
A) dental X-ray - panoramic
B) upper gastrointestinal tract X-ray
C) chest X-ray
D) mammogram
E) X-ray after barium enema
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Describe what changes occur during beta decay.
A) The mass number and atomic number decrease.
B) The mass number and atomic number increase.
C) The mass number is unchanged and the atomic number decreases.
D) The mass number is unchanged and the atomic number increases.
E) The mass number and atomic number do not change.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing particle in the following nuclear equation:
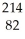 Pb →
Pb → E + ?
E + ?
A) ![]() Bi
Bi
B) ![]() Tl
Tl
C) ![]() Pb
Pb
D) ![]() Pb
Pb
E) ![]() Tl
Tl
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing particle in the following nuclear equation:
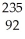 U →
U →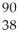 Sr + ? +2
Sr + ? +2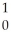 N + 4
N + 4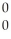 Γ
Γ
A) ![]() Te
Te
B) ![]() Xe
Xe
C) ![]() Xe
Xe
D) ![]() Te
Te
E) ![]() Sr
Sr
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fluorine-18 undergoes positron emission with a half-life of 1.10 × 102 minutes.If a patient is given a 248 mg dose for a PET scan,how long will it take for the amount of fluorine-18 to drop to 83 mg? (Assume that none of the fluorine is excreted from the body. )
A) 99 minutes
B) 1.74 × 102 minutes
C) 1.32 × 102 minutes
D) 3.00 × 102 minutes
E) 2.11 × 102 minutes
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -neutron
A) ![]() γ
γ
B) ![]() p
p
C) ![]() n
n
D) ![]() e
e
E) ![]() He
He
F) ![]() e
e
H) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the positron emission of 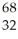 Ge.
Ge.
A) ![]() Ga
Ga
B) ![]() As
As
C) ![]() Zn
Zn
D) ![]() As
As
E) ![]() Ga
Ga
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the alpha decay of 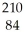 Po.
Po.
A) ![]() Rn
Rn
B) ![]() Pb
Pb
C) ![]() Ra
Ra
D) ![]() Hg
Hg
E) ![]() At
At
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the beta decay of 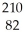 Pb.
Pb.
A) ![]() Pt
Pt
B) ![]() Tl
Tl
C) ![]() Hg
Hg
D) ![]() Bi
Bi
E) ![]() Pb
Pb
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nuclide As-76 has a half-life of 26.0 hours.If a sample of As-76 weighs 344 g,what mass of As-76 remains after 538 minutes?
A) 67.8 g
B) 271 g
C) 144 g
D) 437 g
E) 251 g
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Explain how radiation increases cancer risk.
Correct Answer

verified
Radiation increases cancer risk because ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify the symptom that is not from radiation exposure.
A) increased white cell count
B) increased cancer risk
C) death
D) genetic effects
E) weaker immune systems
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Potassium-40 decays to argon-40 with a half-life of 1.27 × 109 yr.The age of a mineral sample that has a mass ratio of 40Ar to 40K of 3.60 is ________ yr.
A) 3.53 × 108
B) 3.11 × 108
C) 4.49 × 108
D) 1.94 × 109
E) 2.80 × 109
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In addition to a beta particle,what is the other product of beta
decay of 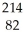 Pb?
Pb?
A) ![]() Hg
Hg
B) ![]() Tl
Tl
C) ![]() Bi
Bi
D) ![]() Po
Po
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 105
Related Exams