Correct Answer

verified
Correct Answer
verified
True/False
Protons and electrons each have a mass of 1 amu.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an atom loses an electron,the resulting particle is called:
A) a proton.
B) an anion.
C) a cation.
D) an isotope.
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
If two atoms each contain different numbers of protons,the atoms must be from different elements.
Correct Answer

verified
Correct Answer
verified
True/False
In the modern periodic table,elements are listed in order of increasing atomic number rather than increasing relative mass.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the atomic symbol for tin?
A) Sn
B) Ti
C) Tn
D) Si
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many neutrons are present in C-14?
A) 14
B) 12
C) 6
D) 8
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The atomic mass of individual atoms of an element may vary.
Correct Answer

verified
Correct Answer
verified
True/False
The plum pudding model proposed that negatively charged electrons were held in a sphere of positive charge.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Isotopes are:
A) atoms of the same element that have different number of neutrons.
B) atoms of the same element that have different number of protons.
C) atoms of the same element that have different number of electrons.
D) atoms of the same element that have the same number of neutrons.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a correct name,symbol combination?
A) beryllium,Be
B) magnesium,Mg
C) iron,I
D) manganese,Mn
E) silicon,Si
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement below accurately describes the contributions of Thomson?
A) ancient Greek philosopher who proposed that matter was continuous
B) created the modern periodic table
C) proposed the modern Atomic Theory
D) discovered the existence of electrons
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the charge on an ion that has an atomic number of 24 and contains 22 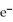 ?
?
A) 2+
B) 2-
C) 1+
D) 1-
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement reflects the results of Rutherford's gold foil experiments?
A) Almost all of the alpha particles were deflected back in the direction from which they came.
B) Almost all of the alpha particles sputtered gold atoms off of the surface of the foil.
C) Almost all of the alpha particles were deflected while passing through the foil.
D) Almost all of the alpha particles passed directly through the foil.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A fictional element named Nivadium is found to have three naturally occurring isotopes with the natural abundances shown here: 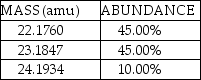 The calculated atomic mass of Nivadium is:
The calculated atomic mass of Nivadium is:
A) 7.61 amu
B) 22.83 amu
C) 23.18 amu
D) 69.55 amu
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The atom is the fundamental building block of everything we hear,feel,see,and experience.
Correct Answer

verified
Correct Answer
verified
True/False
All carbon atoms have exactly 6 protons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a correct name,symbol combination?
A) calcium,Ca
B) gold,Au
C) manganese,Mn
D) chromium,Cr
E) potassium,P
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many protons and neutrons are in Cl-37?
A) 20 protons,17 neutrons
B) 17 protons,37 neutrons
C) 17 protons,20 neutrons
D) 37 protons,17 neutrons
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A fictional element has two naturally occurring isotopes with the natural abundances shown here: 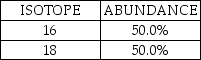 Which statement is TRUE for this element?
Which statement is TRUE for this element?
A) The atomic mass would be 16.
B) The atomic mass would be less than 16.
C) The atomic mass would be 18.
D) The atomic mass would be more than 18.
E) The atomic mass would be 17.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 112
Related Exams