Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the theoretical yield of C when 3 units of A and 10 units of B are reacted in the following generic chemical equation: 2A + 5B → 4C.
A) 3
B) 4
C) 6
D) 8
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the theoretical yield of waffles if you have 6 cups of flour,9 eggs and 2 tbs of oil? Given: 2 cups flour + 3 eggs + 1 tbs oil → 4 waffles
A) 10
B) 12
C) 8
D) 4
E) not enough information
Correct Answer

verified
Correct Answer
verified
True/False
The conversion factor for moles of carbon dioxide to mass of carbon dioxide is: 1 mole CO2 ≡ 44.01 g.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that 4 NH3 + 5 O2 → 4 NO + 6 H2O,when 4.50 mol of H2O are formed,the amount of NO formed is:
A) 1.50 mol.
B) 3.00 mol.
C) 4.50 mol.
D) 6.75 mol.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of NO2 are theoretically produced if we start with 1.20 moles of S and 9.90 moles of HNO3? Reaction: S + 6HNO3 → H2SO4 + 6NO2 + 2H2O
A) 7.20
B) 331
C) 455
D) 786
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is false?
A) The limiting reactant is completely consumed in a chemical reaction.
B) The theoretical yield is the amount of product that can be made based on the amount of limiting reagent.
C) The actual yield is the amount of product actually produced by a chemical reaction.
D) The percent yield = ![]() × 100%.
× 100%.
E) All of the above are true statements.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the balanced equation CO2 + Si → SiO2 + C,if you were to react 1 mole of CO2 with 1 mole of Si,which statement is TRUE?
A) The CO2 is the limiting reactant.
B) The Si is the limiting reactant.
C) The SiO2 is the limiting reactant.
D) You have equal stoichiometric amounts of reactants.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the theoretical yield of a reaction if 25.0 grams of product were actually produced from a reaction that has a 88% yield?
A) 28.4
B) 22.0
C) 3.52
D) 352
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Since heat must be supplied to melt ice,the melting of ice is an endothermic process and so has a positive enthalpy value.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the excess reactant in the following reaction given that you start with 15.5 g of Na2S and 12.1 g CuSO4? Reaction: Na2S + CuSO4 → Na2SO4 + CuS
A) Na2S
B) CuSO4
C) Na2SO4
D) CuS
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of water are needed to react with 2.2 moles of Li2O? Given: Li2O + H2O → 2 LiOH
A) 4.4
B) 1.1
C) 1.5
D) 2.2
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of the excess reactant remain assuming the reaction goes to completion and that you start with 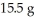 of Na2S and
of Na2S and  CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
A) 0.05
B) 15.45
C) 9.58
D) 5.92
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the limiting reactant for the following reaction given we have 2.6 moles of HCl and 1.4 moles of Ca(OH) 2? Reaction: 2HCl + Ca(OH) 2 → 2H2O + CaCl2
A) Ca(OH) 2
B) HCl
C) H2O
D) CaCl2
E) not enough information
Correct Answer

verified
Correct Answer
verified
True/False
Global warming is due to the greenhouse gases preventing heat from Earth escaping into space.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the excess reactant for the reaction below given that you start with 10.0 grams of Al and 19.0 grams of O2? Reaction: 4Al + 3O2 → 2Al2O3
A) Al
B) O2
C) Al2O3
D) both Al and O2
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the theoretical yield of the reaction below corresponds to 25.3 g and the percent yield of the reaction is known to be reproducibly 81.1%,calculate the actual yield. Given: Li2O + H2O → 2 LiOH
A) 20.5 g
B) 48.9 g
C) 45.8 g
D) 81.1 g
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of water are made from complete reaction of 2.2 moles of oxygen gas with hydrogen gas? Given the reaction: 2H2 + O2 → 2H2O
A) 4.4
B) 1.1
C) 2.2
D) 3.3
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the theoretical yield of the reaction below corresponds to 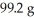 and the actual yield was 60.9 g,calculate the percent yield.
Given: Li2O + H2O → 2 LiOH
and the actual yield was 60.9 g,calculate the percent yield.
Given: Li2O + H2O → 2 LiOH
A) 16.0 %
B) 71.8 %
C) 38.0 %
D) 61.4 %
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The theoretical yield of a reaction is 75.0 grams of product and the actual yield is 42.0g.What is the percent yield?
A) 75.0
B) 56.0
C) 31.5
D) 178
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 115
Related Exams