A) the Pauli exclusion principle.
B) Bohr's equation.
C) Hund's rule.
D) de Broglie's relation.
E) Dalton's atomic theory.
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following is diamagnetic both in its ground state and in all of its excited states?
A) Mg
B) Ne
C) Cu
D) Zn
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ground-state atoms is diamagnetic?
A) Ca
B) As
C) Cu
D) Fe
E) none of these
Correct Answer

verified
Correct Answer
verified
True/False
The frequency of the emitted light from a cesium atom is an intensive property.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electron configuration of a ground-state copper atom is
A) [Ar]4s24d4.
B) [Ar]4s24p63d3.
C) [Ar]4s23d9.
D) [Ar]3d9.
E) [Ar]4s13d10.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The orbital diagram for a ground-state oxygen atom is 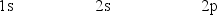
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many unpaired electrons does a ground-state atom of sulfur have?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are there in the 3rd principal energy level (n = 3) of a phosphorus atom?
A) 3
B) 5
C) 6
D) 8
E) 10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete this sentence: Atoms emit visible and ultraviolet light
A) as electrons jump from lower energy levels to higher levels.
B) as the atoms condense from a gas to a liquid.
C) as electrons jump from higher energy levels to lower levels.
D) as they are heated and the solid melts to form a liquid.
E) as the electrons move about the atom within an orbit.
Correct Answer

verified
Correct Answer
verified
Short Answer
The colors of the visible spectrum are blue, green, orange, red, violet, and yellow. Of these colors, _______ has the longest wavelength.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the ground-state electron configuration of a calcium atom?
A) [Ne]3s2
B) [Ne]3s23p6
C) [Ar]4s13d1
D) [Ar]4s2
E) [Ar]3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A single pulse of a laser yields an average of 5.00 * 1018 photons with = 633 nm. If melting ice to water at 0°C requires 6.01 kJ/mol, what is the fewest number of laser pulses need to melt 10.0 g of ice?
A) 3830
B) 3340
C) 38300
D) 2120
E) 212
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element has the following ground-state electron configuration? [Kr]5s24d105p3
A) Sn
B) Sb
C) Pb
D) Bi
E) Te
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
What is the wavelength of radiation that has a frequency of 2.10 * 1014 s -1?
A) 6.30 * 1022 m
B) 7.00 * 102 nm
C) 7.00 * 105 m
D) 1.43 * 10-6 m
E) 3.00 * 108 m
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the total number of electrons possible in the 6s orbital?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many orbitals are allowed in a subshell if the angular momentum quantum number for electrons in that subshell is 3?
A) 1
B) 3
C) 5
D) 7
E) 9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Transition metal elements have atoms or ions with partially filled
A) s subshells.
B) p subshells.
C) d subshells.
D) f subshells.
E) g subshells.
Correct Answer

verified
Correct Answer
verified
Short Answer
Write the ground state electron configuration for Cr.
Correct Answer

verified
[Ar] 4s13d5
Correct Answer
verified
Multiple Choice
The electron in a hydrogen atom falls from an excited energy level to the ground state in two steps, causing the emission of photons with wavelengths of 2624 and 97.2 nm. What is the quantum number of the initial excited energy level from which the electron falls?
A) 2
B) 3
C) 4
D) 6
E) 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the electron in a hydrogen atom falls from its first excited energy level to the ground state energy level, a photon with wavelength is emitted. A proton having this same wavelength would have a velocity of
A) 3.87 m/s.
B) 5990 m/s.
C) 1.21 * 10-7 m/s.
D) 3.26 m/s.
E) 5.99 m/s.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 115
Related Exams