A) 1.
B) 2.
C) 3.
D) 4.
E) None of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Classify the Ca - Cl bond in CaCl2 as ionic, polar covalent, or nonpolar covalent.
A) ionic
B) polar covalent
C) nonpolar covalent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assuming the octet rule is obeyed, how many covalent bonds will an oxygen atom form to give a formal charge of zero?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances will display an incomplete octet in its Lewis structure?
A) CO2
B) Cl2
C) ICl
D) NO
E) SO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules has an atom with an incomplete octet?
A) NF3
B) H2O
C) AsCl3
D) GeH4
E) BF3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a useful guideline for the application of formal charges in neutral molecules?
A) A Lewis structure in which there are no formal charges is preferred.
B) Lewis structures with large formal charges are preferred.
C) The preferred Lewis structure is one in which positive formal charges are on the most electronegative atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of lone electron pairs in the N2 molecule is ___.
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Essay
Write the Lewis structure of ammonia (nitrogen trihydride).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the atoms listed below is the most electronegative?
A) Li
B) Cs
C) P
D) As
E) Ge
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the elements listed below has the greatest electronegativity?
A) Mg
B) Ga
C) Si
D) Ba
E) Pb
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the energy change for the reaction K(g) + Br(g) K+(g) + Br- (g)
Given the following ionization energy (IE) and electron affinity (EA) values 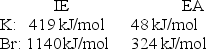
A) -1,092 kJ/mol
B) -95 kJ/mol
C) 95 kJ/mol
D) 1,092 kJ/mol
E) 1,187 kJ/mol
Correct Answer

verified
Correct Answer
verified
Essay
Carbonic acid, H2CO3, is a weak acid that contributes to the taste and produces the carbon dioxide bubbles in all carbonated beverages. Write a Lewis structure for H2CO3,
Correct Answer

verified
Correct Answer
verified
Multiple Choice
BeF42- is called the fluoberyllate ion. The formal charge on the beryllium atom in this ion is
A) -2.
B) -1.
C) 0.
D) +1.
E) +2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis structure for CS2 is:
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is most likely to be an ionic compound?
A) CaCl2
B) CO2
C) CS2
D) SO2
E) OF2
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the hypothetical element X with electron-dot formula 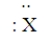 Give the formula for the simplest compound this element forms with chlorine.
Give the formula for the simplest compound this element forms with chlorine.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The total number of lone pairs in NCl3 is
A) 6.
B) 8.
C) 9.
D) 10.
E) 13.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the elements listed below is most likely to exhibit an expanded octet in its compounds?
A) O
B) S
C) Na
D) C
E) N
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bond in which one of the following pairs of atoms would be the most polar?
A) B - C
B) C - N
C) C - O
D) Si - O
E) C - C
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the hypothetical element Z with electron-dot formula 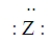 Give the formula for the simplest compound this element forms with chlorine.
Give the formula for the simplest compound this element forms with chlorine.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 118
Related Exams