A) CH3OH
B) COCl2
C) CH3CO2-
D) CO32-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the bond angles in the following molecular model of BCl3? 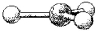
A) less than 109.5°
B) 109.5°
C) less than 120° but greater than 109.5°
D) 120°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecular model of NO3- is shown below.Based on the best Lewis electron-dot structure for NO3- and formal charge considerations,what is the predicted N-O bond order for each N-O bond? 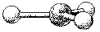
A) 1
B) 1) 33
C) 1) 5
D) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound ClF contains
A) ionic bonds.
B) nonpolar covalent bonds.
C) polar covalent bonds with partial negative charges on the F atoms.
D) polar covalent bonds with partial negative charges on the Cl atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following elements,which has the highest electronegativity?
A) P
B) S
C) Sc
D) As
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which orbital hybridization is associated with a tetrahedral charge cloud arrangement?
A) sp
B) sp2
C) sp3
D) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The phosphorus atom in PCl3 would be expected to have a
A) partial positive (δ+) charge.
B) partial negative (δ-) charge.
C) 3+ charge.
D) 3- charge.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the angle between adjacent sp3 hybrid orbitals?
A) 90°
B) 109.5°
C) 120°
D) 180°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electronegativity is 2.1 for H and 1.8 for Si.Based on these electronegativities,SiH4 would be expected to
A) be ionic and contain H- ions.
B) be ionic and contain H+ ions.
C) have polar covalent bonds with a partial negative charges on the H atoms.
D) have polar covalent bonds with a partial positive charges on the H atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following ball-and-stick molecular model is a representation of the amino acid alanine (unshaded spheres = H) .Only the connections between atoms are shown;multiple bonds and nonbonded electrons are not indicated.  -In order to complete an electron-dot structure of alanine,the nitrogen atom needs
-In order to complete an electron-dot structure of alanine,the nitrogen atom needs
A) 1 additional bond and 1 nonbonded pair of electrons.
B) 1 additional bond and 2 nonbonded pairs of electrons.
C) 1 nonbonded pair of electrons.
D) 2 nonbonded pairs of electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecular compound that obeys the octet rule in which all atoms have a zero formal charge is
A) BaCl2
B) BrF3
C) NCl3
D) XeF4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecular orbital resembles a p-orbital?
A) σ
B) σ*
C) π
D) π*
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule contains the most polar bonds?
A) CF4
B) CO2
C) CN-
D) CH4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the geometry around the central atom in the following molecular model of XeF4? 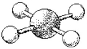
A) trigonal bipyramidal
B) octahedral
C) square pyramidal
D) square planar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the nitrogen atom?
A) sp
B) sp2
C) sp3
D) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which drawing represents the molecular orbital containing the highest energy electrons in the H2 molecule in its ground state?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the best Lewis structure for NO+,what is the formal charge on the N atom?
A) -1
B) 0
C) +1
D) +2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Elements that can accommodate more than eight electrons in their valence shell occur only in periodic table period
A) 2 or lower.
B) 3 or lower.
C) 4 or lower.
D) 5 or lower.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound ICl contains
A) ionic bonds.
B) nonpolar covalent bonds.
C) polar covalent bonds,with partial negative charges on the Cl atoms.
D) polar covalent bonds,with partial negative charges on the I atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are in the valence shell of I in IF4-?
A) 8
B) 10
C) 12
D) 14
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 232
Related Exams