A) -431 kJ
B) -3.14 kJ
C) +3.14 kJ
D) +431 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The net reaction is represented by
A) arrow A.
B) arrow B.
C) arrow C.
D) line F.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which thermodynamic function is most related to disorder and probability?
A) enthalpy
B) internal energy
C) entropy
D) heat capacity
Correct Answer

verified
Correct Answer
verified
Multiple Choice
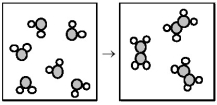 -What are the signs of ΔH and ΔS for the reaction represented in the above drawing?
-What are the signs of ΔH and ΔS for the reaction represented in the above drawing?
A) ΔH = +,ΔS = +
B) ΔH = +,ΔS = -
C) ΔH = -,ΔS = +
D) ΔH = -,ΔS = -
Correct Answer

verified
Correct Answer
verified
Short Answer
When the reaction below is performed in a water bath,will the temperature of the water bath increase or decrease? 432 kJ + A + 2 B → 3 C
Correct Answer

verified
Correct Answer
verified
Short Answer
Kinetic energy increases with increasing ________ and increasing ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heat of vaporization of water at 100°C is 40.66 kJ/mol.Calculate the quantity of heat that is absorbed/released when 9.00 g of steam condenses to liquid water at 100°C.
A) 20.3 kJ of heat are absorbed.
B) 20.3 kJ of heat are released.
C) 81.3 kJ of heat are absorbed.
D) 81.3 kJ of heat are released.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 298 K the average kinetic energy is the same for H2,He,and N2.The gas with the highest average velocity is
A) H2.
B) He.
C) N2.
D) All have the same average velocity.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) from 35.0°C to gaseous CCl4 76.8°C (the normal boiling point for CCl4) ? The specific heat of CCl4(l) is 0) 857 J/(g ∙°C) ,its heat of fusion is 3.27 kJ/mol,and its heat of vaporization is 29.82 kJ/mol.
A) 0) 896 kJ
B) 1) 43 kJ
C) 5) 74 kJ
D) 6) 28 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
He gas is contained in a one-liter flask that is connected to an empty one-liter flask with a closed stopcock between the two flasks.When the stopcock is opened some of the He enters the evacuated flask.For this system
A) ΔH is negative and ΔS is positive
B) ΔH is zero and Δ△S is positive
C) ΔH is zero and ΔS is negative
D) ΔH is positive and ΔS is negative
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Methanol can be produced from carbon monoxide and hydrogen with suitable catalysts: CO(g) + 2 H2(g) → CH3OH(l) at 25°C ΔH° = -128.1 kJ and ΔS° = -332 J/K Find ΔG° at 25°C.
A) -157.2 kJ
B) -29.1 kJ
C) 98.9 kJ
D) 157.2 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following drawing is a representation of a reaction for which ΔH° = +62 kJ.This reaction is likely to be 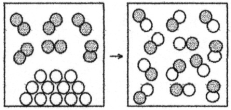
A) nonspontaneous at all temperatures.
B) nonspontaneous at low temperatures and spontaneous at high temperatures.
C) spontaneous at low temperatures and nonspontaneous at high temperatures.
D) spontaneous at all temperatures.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Water has an unusually high
A) electrical conductivity.
B) heat of combustion.
C) heat of formation.
D) specific heat.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a state function?
A) altitude
B) heat
C) internal energy
D) volume
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much heat is absorbed/released when 20.00 g of NH3(g) reacts in the presence of excess O2(g) to produce NO(g) and H2O(l) according to the following chemical equation? 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(l) ΔH° = +1168 kJ
A) 342.9 kJ of heat are absorbed.
B) 342.9 kJ of heat are released.
C) 1372 kJ of heat are absorbed.
D) 1372 kJ of heat are released.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 2CH4 (g) + 3 Cl2 (g) → 2 CHCl3 (l) + 3 H2 (g) ,ΔH° = -118.6 kJ. ΔH°f = -134.1 kJ/mol for CHCl3 (l) .Find ΔH°f for CH4 (g) .
A) -193.4 kJ/mol
B) -74.8 kJ/mol
C) 74.8 kJ/mol
D) 193.4 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At constant pressure for the reaction shown below,what can be said about PΔV and ΔE? N2(g) + 3 H2(g) → 2 NH3(g) ΔH° = - 92.2 kJ
A) PΔV > 0 and ΔE > -92.2 kJ
B) PΔV > 0 and ΔE < -92.2 kJ
C) PΔV < 0 and ΔE > -92.2 kJ
D) PΔV < 0 and ΔE < -92.2 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is not a spontaneous process?
A) combustion of gasoline to produce carbon dioxide and water
B) diffusion of perfume in a room
C) dissolution of sodium chloride in water
D) freezing of water at 1°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetylene torches utilize the following reaction:
2 C2H2(g) + 5 O2(g) → 4 CO2(g) + 2 H2O(g)
Use the given standard enthalpies of formation to calculate ΔH° for this reaction. 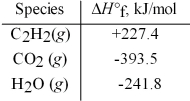
A) 2512.4 kJ
B) 1256.2 kJ
C) -1256.2 kJ
D) -2512.4 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a process at constant pressure,
A) ΔE = w and q = 0.
B) ΔE = q and w = 0.
C) ΔE = ΔH.
D) ΔH = q.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 163
Related Exams