A) 1.44 × 10-22 mol L-1
B) 0.0100 mol L-1
C) 8.35 × 1019 mol L-1
D) 6.94 × 1021 mol L-1
Correct Answer

verified
Correct Answer
verified
True/False
Equilibrium reactions are noted by a single straight arrow for a yield sign.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following equilibrium constants-reaction types is INCORRECT?
A) 1.4 × 1083 equilibrium reaction-goes to completion.
B) 1.6 × 10-23 equilibrium reaction-does not occur.
C) 1.8 × 10-5 equilibrium reaction-more reactants than products at equilibrium.
D) 1.0 equilibrium reaction-equal amounts of products and reactants.
E) 3.2 × 103 equilibrium reaction-more reactants than products at equilibrium.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium constant,Kp,equals 3.40 for the isomerization reaction: Cis-2-butene ⇌ trans-2-butene If a flask initially contains 0.250 bar of cis-2-butene and 0.165 bar of trans-2-butene,what is the equilibrium pressure of each gas?
A) P(cis-2-butene) = 0.0485 bar and P(trans-2-butene) = 0.165 bar
B) P(cis-2-butene) = 0.0458 bar and P(trans-2-butene) = 0.156 bar
C) P(cis-2-butene) = 0.0735 bar and P(trans-2-butene) = 0.250 bar
D) P(cis-2-butene) = 0.0943 bar and P(trans-2-butene) = 0.321 bar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction occurring at 960 K: CO2(g) + H2(g) ⇌ CO(g) + H2O(g) At equilibrium,the concentrations of reactants and products are: [CO2] = 0.0400 M,[H2] = 0.0220 M,[CO] = 0.0240 M,and [H2O] = 0.0190 M.This equilibrium is perturbed by adding CO2(g) to the system such that when a new equilibrium is reached,the concentration of CO2 is 0.050 M.What is the concentration of H2(g) at this equilibrium?
A) 0.050 M
B) 0.482 M
C) 0.462 M
D) 0.300 M
E) 0.0504 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: 3 Fe(s) + 4 H2O(g) ⇌ Fe3O4(s) + 4 H2(g) Write the expression for Kp.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: 2 SO2(g) + O2(g) ⇌ 2 SO3(g) ,ΔrH° = -196.6 kJ/mol The equilibrium is displaced to the left if:
A) some sulfur trioxide is removed
B) the temperature is raised
C) some sulfur dioxide is added
D) the pressure is raised
E) the temperature is lowered
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the equilibrium constant expression for the reaction: 3 Sn(s) + 4 HNO3(aq) + H2O(l) ⇌ 3 H2SnO3(s) + 4 NO(g)
A) Kc = ![]()
B) Kc = ![]()
C) Kc = ![]()
D) Kc = ![]()
E) Kc = ![]()
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
At a certain temperature the equilibrium constant,Kc,equals 0.11 for the reaction: 2 ICl(g) ⇌ I2(g) + Cl2(g) What is the equilibrium concentration of ICl if 0.45 mol of I2 and 0.45 mol of Cl2 are initially mixed in a 2.0 L flask?
A) 0.14 mol L-1
B) 0.17 mol L-1
C) 0.27 mol L-1
D) 0.34 mol L-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the equilibrium system: N2O4(g) ⇌ 2 NO2(g) for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assume that 1 mole of N2O4 and 2 moles of NO2 are introduced into a 5.0 liter container.What will be the equilibrium value of [N2O]?
A) 0.928 M
B) 0.0822 M
C) 0.358 M
D) 0.379 M
E) 0.042 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction: POCl3(g) ⇌ POCl(g) + Cl2(g) with Kc = 0.450 A sample of pure POCl3(g) was placed in a reaction vessel and allowed to decompose according to the above reaction.At equilibrium,the concentrations of POCl(g) and Cl2(g) were each 0.150 M.What was the initial concentration of POCl3(g) ?
A) 0.225 M
B) 0.200 M
C) 0.633 M
D) 0.483 M
E) 0.350 M
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Consider the equilibrium system: N2O4(g) ⇌ 2 NO2(g) for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assuming that the total pressure inside the container is 1.00 atm at equilibrium and that initially only N2O4 was present inside the container,compute 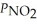 At equilibrium.
At equilibrium.
A) 0.398 atm
B) 0.113 atm
C) 0.602 atm
D) 0.285 atm
E) 0.715 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following hypothetical equilibrium reaction:
A2(g) + B2(g) ⇌ 2 AB(g) where Kc = 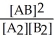 The equilibrium constant for the reaction: 2 A2(g) + 2 B2(g) ⇌ 4 AB(g) is ________.
The equilibrium constant for the reaction: 2 A2(g) + 2 B2(g) ⇌ 4 AB(g) is ________.
A) ![]()
B) Kc4
C) ![]()
D) ![]()
E) Kc2
Correct Answer

verified
E
Correct Answer
verified
True/False
In a system in equilibrium,two opposing reactions occur at equal rates.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the equilibrium system: N2O4(g) ⇌ 2 NO2(g) for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assuming that the total pressure inside the container is 10 atm at equilibrium and that initially only N2O4 was present inside the container,compute 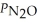 At equilibrium.
At equilibrium.
A) 7.98 atm
B) 8.88 atm
C) 1.12 atm
D) 1.01 atm
E) 8.99 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following chemical equilibrium,Kp = 4.6 × 10-14 at 25 °C,find the value of Kc for this reaction at 25 °C. 2 Cl2(g) + 2 H2O(g) ⇌ 4 HCl(g) + O2(g)
A) Kc = 1.9 × 10-15
B) Kc = 2.2 × 10-14
C) Kc = 1.1 × 10-12
D) Kc = 9.4 × 10-14
E) Kc = 4.6 × 10-14
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction CO(g) + 3 H2(g) ⇌ H2O(g) + CH4(g) ,Kc = 190 at 1000 K.If a vessel is filled with these gases such that the initial concentrations are [CO] = 0.025 M,[H2] = 0.045 M,[H2O] = 0.025,M and [CH4] = 0.046 M,in which direction will a reaction occur and why?
A) toward products because Qc = 0.17
B) toward reactants because Qc = 0.0029
C) toward products because Qc = 0.35
D) toward reactants because Qc = 505
E) it is at equilibrium because Qc = 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement.
A) A certain amount of energy,called the activation energy,must be available if a reaction is to take place.
B) A reversible chemical reaction is one in which equilibrium is never established due to the constant decomposition of the products.
C) When the rate of the reverse reaction equals the rate of the forward reaction,equilibrium has been established.
D) Changes in temperature will change the value of an equilibrium constant.
E) Chemical equilibrium is a dynamic equilibrium.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction CO(g) + 3 H2(g) ⇌ H2O(g) + CH4(g) ,Kc = 190 at 1000 K.If a vessel is filled with these gases such that the initial concentrations are [CO] = 0.036 M,[H2] = 0.045 M,[H2O] = 0.020,M and [CH4] = 0.031 M,in which direction will a reaction occur and why?
A) toward products because Qc = 0.38
B) toward reactants because Qc = 0.24
C) toward products because Qc = 4.1
D) toward reactants because Qc = 61
E) it is at equilibrium because Qc = 190
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the equilibrium constant expression for the following reaction: 2 KI(aq) + H2O2(aq) ⇌ 2 KOH(aq) + I2(aq)
A) Kc = ![]()
B) Kc = [I2]
C) Kc = [I2]2
D) Kc = ![]()
E) Kc = ![]()
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 118
Related Exams