A) The neutron is nearly massless.
B) The neutron is only slightly more massive than the proton.
C) The discovery required the use of ultrafast computers.
D) The neutron lacks an electrical charge.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best explains what is happening when an atom emits light?
A) An electron is dropping from a higher to a lower energy level with the difference in energy between the two being emitted as light energy.
B) A proton is undergoing a nuclear change in the nucleus and is emitting a high energy light wave in the process.
C) An electron is jumping from a low energy state to a high energy state with the difference in energy being converted to light energy.
D) Heat energy is speeding up the orbit of the electrons and the resulting Doppler shift causes light to be emitted from the electrons.
E) Heat energy is converting a neutron into a proton and an electron, which is ejected into the orbit of the nucleus, releasing light energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following atoms is the largest?
A) Ba
B) Sr
C) Ca
D) Mg
E) Be
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following statement describes which subatomic particle best? It does not have an electrical charge.
A) an electron
B) a proton
C) a neutron
D) A and B
E) B and C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a property of an electron?
A) charge changes depending on mass
B) fundamental component of atoms
C) attracted towards positively charge electrical plates
D) weighs approximately 9.1 × 10-31 kg
E) has a negative charge
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Does a shell have to contain electrons in order to exist?
A) A shell is a form of energy that requires electrons in order to exist.
B) A shell is just a region of space which may or may not contain electrons.
C) A shell is just a conceptual model, hence, it doesn't really exist with or without the electron.
D) Two of the above are reasonable answers.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an element has 18 protons and 20 neutrons and 18 electrons, which expression correctly identifies the element?
A) argon-38
B) argon-18
C) argon-20
D) calcium-38
E) calcium-20
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How can a hydrogen atom, which has only one electron, have so many spectral lines?
A) The electron is able to move at various speeds.
B) The protons in the nucleus are also giving off various light frequencies.
C) Because there is more than one energy level in a hydrogen atom.
D) The atomic radius of the hydrogen atom is relatively large.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rutherford assumed that the atomic nucleus was positively charged because ________.
A) electrons were already established to be negatively charged
B) the alpha particles that ricocheted off of the nucleus are also positively charged
C) he had a very optimistic outlook on life
D) Two of the above are reasonable answers
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following by the relative amounts of energy, from most energetic to least:
A) gamma rays, blue light, red light, radio waves
B) gamma rays, radio waves, blue light, red light
C) radio waves, gamma rays, blue light, red light
D) blue light, red light, radio waves, gamma rays
E) red light, radio waves, blue light, gamma rays
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An electron in the outermost shell of which group 1 element experiences the greatest effective nuclear charge?
A) Sodium, Na
B) Potassium, K
C) Rubidium, Rb
D) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true about Bohr's planetary model of the atom?
A) The electrons orbit around the nucleus.
B) It is a physical model.
C) The energy difference between the orbits is continuous.
D) The electrons smoothly move from one orbit to the next.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the main difference between a conceptual model and a physical model?
A) A physical model represents the shape and form while a conceptual model describes how a system behaves.
B) A conceptual model represents the shape and form while a physical model describes how a system behaves.
C) Physical models can only be used to represent the real world.
D) Conceptual models can only be used to describe concepts.
E) Physical models and conceptual models can be used to describe the same things.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does the wave model of electrons orbiting the nucleus account for the fact that the electrons can have only discrete energy values?
A) Electrons are only able to vibrate at particular frequencies.
B) When an electron wave is confined, it is reinforced only at particular frequencies.
C) The energy values of an electron only occur where its wave properties and probability clouds are mutually reinforcing.
D) The wave model accounts for the types of orbitals an electron may occupy, not it's energy levels.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What does the following element description actually mean? 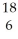 O
O
A) an oxygen atom with 6 protons and 12 neutrons
B) an oxygen atom with 6 neutrons and 12 protons
C) 6 oxygen atoms with 18 neutrons
D) 18 oxygen molecules with 6 neutrons each
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the above has the highest frequency?
A) a
B) b
C) c
D) d
E) e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you remove two protons and two electrons from a sulfur atom (S) , what new element is formed?
A) Si
B) Si+2
C) Al+2
D) Al
E) Ar-2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is the term photon related to the term quantum?
A) A quantum of light is actually one photon.
B) A quantum of photons is equal to the wavelength.
C) A quantum is a particle of light while a photon is a wave of light.
D) A quantum is a particle of light exactly one photon long.
E) A quantum is a wave of light while a packet of quanta equals one photon.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a neutral element has 8 neutrons and 7 electrons, which expression correctly identifies the element?
A) ![]() N
N
B) ![]() O
O
C) ![]() O
O
D) ![]() O
O
E) cannot tell from information given
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is said to be quantized?
A) the number of eggs in an egg carton
B) the amount of water in a glass
C) the volume of air in a balloon
D) the amount of time in an hour
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 146
Related Exams