A) J.J. Thompson's cathode ray deflection experiments
B) E. Rutherford's gold foil experiments
C) Avogadro's number experiments
D) Franklin's kite experiments
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atomic orbital ________.
A) is the path of an electron
B) is a region of space
C) can hold up to four electrons
D) is always spherical
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If two protons and two neutrons are removed from the nucleus of the oxygen-16 isotope, a nucleus of which element remains?
A) nitrogen-12
B) carbon-12
C) neon-18
D) carbon-14
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a neutral element has the following chemical symbol, how many electrons does it have? 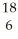 O
O
A) 6
B) 18
C) 12
D) 24
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following atoms is the smallest?
A) Br
B) Ga
C) As
D) Ca
E) K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What property of an electron makes it possible to use electron microscopes?
A) They can be focused using smaller lenses.
B) They have shorter wavelengths than visible light.
C) They are electrically charged.
D) They can be generated with electricity.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these shows light waves in order of increasing energy?
A) Radio < Microwave < Infrared < Ultraviolet < Visible
B) Visible < Ultraviolet < Microwave < Infrared < Radio
C) Ultraviolet < Visible < Infrared < Microwave < Radio
D) Radio < Microwave < Infrared < Visible < Ultraviolet
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a form of electromagnetic radiation?
A) sound waves
B) gamma rays
C) microwaves
D) infrared
E) X-rays
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An electron in an outermost shell has more energy than one in an innermost shell because ________.
A) it is surrounded by more electrons
B) it is farther from the nucleus
C) its principle quantum number is higher
D) It actually has less energy because of inner-shell shielding
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Another interesting periodic trend is the density of the elements. We find that osmium, Os (atomic number 76) has the greatest density of all elements, and, with some exceptions, the closer an element is positioned to osmium, the greater its density. Based upon this trend, list the following elements in order of increasing density: copper, Cu, gold, Au, platinum, Pt, and silver, Ag.
A) copper, Cu < gold, Au < platinum, Pt < silver, Ag
B) copper, Cu < silver, Ag < gold, Au < platinum, Pt
C) copper, Cu < silver, Ag < platinum, Pt < gold, Au
D) platinum, Pt < gold, Au < silver, Ag < copper, Cu
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which color of light comes from the higher energy transition, red or blue?
A) Blue is a higher frequency and therefore corresponds to a lower energy level transition.
B) Blue is a higher frequency and therefore corresponds to a higher energy level transition.
C) Red is a higher frequency and therefore corresponds to a lower energy level transition.
D) Red is a higher frequency and therefore corresponds to a higher energy level transition.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a neutral element has the following chemical symbol, how many electrons does it have? 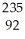 U
U
A) 92
B) 82
C) 235
D) 143
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the above has the highest wavelength?
A) a
B) b
C) c
D) d
E) e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why can't we see atoms?
A) We see with light energy and the wavelength of light is larger than the atom and is not reflected.
B) Atoms are invisible.
C) We cannot see things that are microscopic.
D) We see with light energy but the atoms absorb all the light and therefore there are no reflections.
E) Atoms do not interact with light energy and therefore we are unable to observe them with light.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In some instances electromagnetic radiation behaves like a wave. In other instances electromagnetic radiation behaves more like a particle. Which behavior more accurately describes the true nature of electromagnetic radiation?
A) Particle behavior more accurately describes the true nature of electromagnetic radiation.
B) Electromagnetic radiation behaves both as a wave or as a particle depending on the circumstance.
C) Wave behavior more accurately describes the true nature of electromagnetic radiation.
D) There is no circumstance in which electromagnetic radiation can be described as either portraying particle or wave behavior.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What does the following element description actually mean? hydrogen-2
A) a hydrogen with one neutron and one proton
B) a hydrogen with two neutrons
C) a hydrogen with two protons
D) a molecule of hydrogen gas
E) two hydrogen atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about electrons is true?
A) Electrons have a negative charge.
B) Electrons are particles.
C) Electrons behave like waves.
D) Electrons in atoms can be excited by light energy.
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Does it make sense to say that a textbook is about 99.9 percent empty space?
A) No. A textbook is a solid and thus is quite dense. Therefore it is not 99.9 percent empty space.
B) No. Only gases are considered to be 99.9 percent empty space. Liquids and solids are not.
C) Yes. A textbook like all material things is made up of atoms, which are considered to be 99.9 percent empty space.
D) No. A textbook could only be considered to be 99.9 percent empty space if it were combusted.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A beam of protons and a beam of neutrons of the same energy are both harmful to living tissue. The beam of neutrons, however, is less harmful. Why?
A) Neutrons are much smaller and lighter than protons and would do less damage.
B) Neutrons travel at reduced speed compared to the speed at which protons travel.
C) Neutrons carry no electric charge and thus have a greater likelihood of passing through the tissue.
D) All of the above are reasons why neutrons are less harmful.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Since atoms are mostly empty space, why don't objects pass through one another?
A) The electrons on the atoms repel other electrons on other atoms when they get close.
B) The nucleus of one atom repels the nucleus of another atom when it gets close.
C) The nucleus of one atom attracts the nucleus of a neighboring atom to form a barrier.
D) The electrons of one attracts the nucleus of a neighboring atom to form a barrier.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 146
Related Exams