A) 25.6
B) 64.0
C) 128
D) 1280
E) 256
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for H2SO3 is ________.
A) sulfuric acid
B) sulfurous acid
C) hydrosulfuric acid
D) hydrosulfic acid
E) sulfur hydroxide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The elements in groups 1A, 6A, and 7A are called ________, respectively.
A) alkaline earth metals, halogens, and chalcogens
B) alkali metals, chalcogens, and halogens
C) alkali metals, halogens, and noble gases
D) alkaline earth metals, transition metals, and halogens
E) halogens, transition metals, and alkali metals
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many protons are there in one atom of 71Ga?
A) 40
B) 70
C) 71
D) 31
E) 13
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Vanadium has two naturally occurring isotopes, 50V with an atomic mass of 49.9472 amu and 51V with an atomic mass of 50.9440. The atomic weight of vanadium is 50.9415. The percent abundances of the vanadium isotopes are ________% 50V and ________% 51V.
A) 0.25, 99.75
B) 99.75, 0.25
C) 49, 51
D) 1.0, 99
E) 99, 1.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for HClO3 is ________.
A) hydrochloric acid
B) perchloric acid
C) chloric acid
D) chlorous acid
E) hydrochlorous acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxygen is a ________ and nitrogen is a ________.
A) metal, metalloid
B) nonmetal, metal
C) metalloid, metalloid
D) nonmetal, nonmetal
E) nonmetal, metalloid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Magnesium and sulfur form an ionic compound with the formula ________.
A) MgS
B) Mg2S
C) MgS2
D) Mg2S2
E) Mg2S3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which metal forms cations of differing charges?
A) K
B) Cs
C) Ba
D) Al
E) Sn
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fluorine forms an ion with a charge of ________.
A) 1-
B) 1+
C) 2+
D) 3+
E) 3-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element ________ is the most similar to sodium in chemical and physical properties.
A) Mg
B) Br
C) N
D) K
E) Sr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair of elements is most apt to form an ionic compound with each other?
A) barium, bromine
B) calcium, sodium
C) oxygen, fluorine
D) sulfur, fluorine
E) nitrogen, hydrogen
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for HNO2 is ________.
A) nitrous acid
B) nitric acid
C) hydrogen nitrate
D) hyponitrous acid
E) pernitric acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
There are ________ protons, ________ neutrons, and ________ electrons in 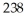 U+5.
U+5.
A) 146, 92, 92
B) 92, 146, 87
C) 92, 146, 92
D) 92, 92, 87
E) 146, 92, 146
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for Al2O3 is ________.
A) aluminum oxide
B) dialuminum oxide
C) dialuminum trioxide
D) aluminum hydroxide
E) aluminum trioxide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following basic forces is so small that it has no chemical significance?
A) weak nuclear force
B) strong nuclear force
C) electromagnetism
D) gravity
E) Coulomb's law
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass number of an atom of 14C is ________.
A) 6
B) 20
C) 8
D) 14
E) 10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Potassium forms an ion with a charge of ________.
A) 2+
B) 1-
C) 1+
D) 2-
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The proper formula for the hydronium ion is ________.
A) H-
B) OH-
C) N3-
D) H3O+
E) NH4+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulfur forms an ion with a charge of ________.
A) 2+
B) 2-
C) 3+
D) 6-
E) 6+
Correct Answer

verified
Correct Answer
verified
Showing 201 - 220 of 250
Related Exams