A) is one with more than one solute
B) is one that has been heated
C) is one with a higher concentration than the solubility
D) must be in contact with undissolved solid
E) exists only in theory and cannot actually be prepared
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is prepared by dissolving calcium chloride in water and diluting to 500.0 mL. If this solution contains 44 ppm chloride ions, the concentration of calcium ions is ________ ppm.
A) 44
B) 88
C) 22
D) 11
E) 500
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molality of a 10.0% (by mass) aqueous solution of hydrochloric acid.
A) 2.40 m
B) 87.4 m
C) 0.791 m
D) 1.20 m
E) The density of the solution is needed to solve the problem.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
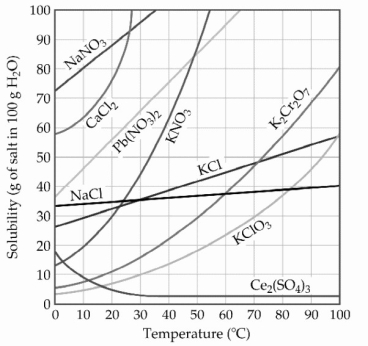 -A sample of potassium chlorate (15.0 g) is dissolved in 201 g of water at 70 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is ________.
-A sample of potassium chlorate (15.0 g) is dissolved in 201 g of water at 70 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is ________.
A) hydrated
B) miscible
C) saturated
D) unsaturated
E) supersaturated
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The concentration of urea (MW = 60.0 g/mol) in a solution prepared by dissolving 16 g of urea in 39 g of H2O is ________ molal.
A) 96
B) 6.8
C) 0.68
D) 6.3
E) 0.11
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following liquids will have the lowest freezing point?
A) pure H2O
B) aqueous glucose (0.050 m)
C) aqueous CoI2 (0.030 m)
D) aqueous FeI3 (0.030 m)
E) aqueous NaI (0.030 m)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molarity of phosphoric acid (H3PO4) in a 38.4% (by mass) aqueous solution.
A) 0.115 m
B) 0.206 m
C) 0.0514 m
D) 0.103 m
E) The density of the solution is needed to solve the problem.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution contains 30 ppm of benzene. The density of the solution is 1.00 g/mL. This means that ________.
A) there are 30 mg of benzene in 1.0 L of this solution
B) 100 g of the solution contains 30 g of benzene
C) 100 g of the solution contains 30 mg of benzene
D) the solution is 30% by mass of benzene
E) the molarity of the solution is 30 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is prepared by dissolving 15.0 g of NH3 in 250.0 g of water. The density of the resulting solution is 0.974 g/mL. The molality of NH3 in the solution is ________ m.
A) 0.00353
B) 0.882
C) 60.0
D) 3.24
E) 3.53
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is prepared by adding 1.10 mol of KCl to 889 g of water. The concentration of KCl is ________ molal.
A) 1.24 × 10-3
B) 808
C) 0.808
D) 978
E) 1.24
Correct Answer

verified
Correct Answer
verified
True/False
The value of the boiling-point-elevation constant (Kb)depends on the identity of the solvent.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molality of a 27.0% (by mass) aqueous solution of nitric acid.
A) 4.56
B) 370
C) 1.51
D) 2.28
E) The density of the solution is needed to solve the problem.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances is more likely to dissolve in water?
A) HOCH2CH2OH
B) CHCl3
C) O ![]() CH3(CH2) 9CH
CH3(CH2) 9CH
D) CH3(CH2) 8CH2OH
E) CCl4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is prepared by dissolving 2.60 g of a strong electrolyte (formula weight = 101 g/mol) in enough water to make 1.00 L of solution. The osmotic pressure of the solution is 1.25 atm at 25.0 °C. What is the van't Hoff factor (i) for the unknown solute?
A) 0
B) 0.99
C) 1.98
D) 2.98
E) 0.630
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the freezing point (°C) of a solution prepared by dissolving 11.3 g of Ca(NO3) 2 (formula weight = 164 g/mol) in 115 g of water? The molal freezing point depression constant for water is 1.86 °C/m.
A) -3.34
B) -1.11
C) 3.34
D) 1.11
E) 0.00
Correct Answer

verified
Correct Answer
verified
Short Answer
A solution contains 150.8 grams of NaCl in 678.3 grams of water. Calculate the vapor pressure lowering (in torr)of the solution at 25.0 °C. (Note: The vapor pressure of pure water at 25.0 °C is 23.76 torr.)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The concentration of HCl in a solution that is prepared by dissolving 5.5 g of HCl in 200 g of C2H6O is ________ molal.
A) 27.5
B) 7.5 × 10-4
C) 3.3 × 10-2
D) 0.75
E) 1.3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the mole fraction of HCl in a 7.20% (by mass) aqueous solution.
A) 0.0369
B) 0.0383
C) 0.0739
D) 0.0185
E) The density of the solution is needed to solve the problem.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is prepared by dissolving 6.00 g of an unknown nonelectrolyte in enough water to make 1.00 L of solution. The osmotic pressure of this solution is 0.750 atm at 25.0 °C. What is the molecular weight (g/mol) of the unknown solute?
A) 16.4
B) 196
C) 110
D) 30.6
E) 5.12 × 10-3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
 -A sample of potassium nitrate (49.0 g) is dissolved in 101 g of water at 100 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and a small amount of precipitate is observed. This solution is ________.
-A sample of potassium nitrate (49.0 g) is dissolved in 101 g of water at 100 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and a small amount of precipitate is observed. This solution is ________.
A) hydrated
B) placated
C) saturated
D) unsaturated
E) supersaturated
Correct Answer

verified
Correct Answer
verified
Showing 141 - 160 of 160
Related Exams